Waiting for the app-ocalypse: Will toughening FDA regulations disconnect the medical smartphone app industry?
July 22, 2010
by Brendon Nafziger, DOTmed News Associate Editor
This report originally ran in the June 2010 edition of DOTmed Business News.
About a year and a half ago, the U.S. Food and Drug Administration made a decision that many in the booming smartphone medical app industry regard as menacing.
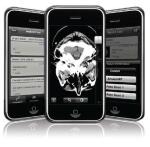
Shortly after the Apple Store launched, the agency told Apple to remove an image viewer produced by the Cleveland-based MIMVista from its virtual shelves. The viewer, Mobile MIM, earned the dubious distinction of being among the first radiology iPhone apps ever taken out of circulation by the agency.
In January, the FDA did something to disturb industry-watchers even more: it rejected the second of MIMVista's so-called fast-track, or 510 (k), applications, requiring the company to go through the lengthy, and expensive, premarket approval before the product could ever be sold by the Apple Store.
Of course, this was an isolated case. Mostly, smartphone users with a medical bent have been in clover. According to a recent MobiHealthNews industry survey, there are around 5,820 medical, health or fitness-themed apps for devices such as the iPhone, iPod Touch and the various Google-run Android platforms. These apps range from iTriage, which lets users check wait times at nearby hospital emergency rooms, to Epocrates, an incredibly popular guide for drug interactions for doctors, and the sundry "wellness" apps that help people schedule workouts and plan their diets. Surveys have shown between two-thirds to three-quarters of doctors own and use some sort of smartphone and often carry the device on rounds.
Yet the news of Mobile MIM's plight coupled with signs of a tougher FDA regulatory attitude compels many to wonder whether they face a brewing storm: will the FDA soon clamp down on the thousands of health care smartphone apps? Will the agency focus mostly on radiology viewers - where there is clear potential for dangerous misreadings by rushed radiologists on tiny screens - or is just the fear of coming regulations going to, in the end, do the most harm?
Light touch
After the initial pulling of the Mobile MIM, few app companies announced being contacted by the FDA, according to most reports. In fact, regulations have actually been fairly light, observed Bradley Merrill Thompson, partner at Epstein Becker and Green, P.C. in Washington D.C. and counsel for EBG Advisors.
"I think the FDA, to their credit, has been holding back to study what's going on, to try to understand the hardware and software and decide what they want to do based on risk," Thompson said.
An authority on medical device law, Thompson literally wrote the book on the subject, "FDA Regulation of Medical Devices," published by Interpharm Press in 1995. He has also testified before Congress on FDA rulemaking.
Planning ahead
Nonetheless, the FDA is taking a closer look at the industry, Thompson said. But even with tougher scrutiny, he thinks the field will remain extremely attractive to companies. Simply put, health care is a good place to be, even with - or in some cases due to - regulatory hassles.
In one of a series of articles published in MobiHealthNews about FDA regulations of smartphone apps, Thompson cited a Thomson Reuters study that showed medical device makers enjoying an average of five-year gross margins of 59 percent, compared with about 46 percent for the Standard & Poor 500. And a Deloitte Center for the Edge study also cited by Thompson found that unlike most of the economy, which only provides one quarter of the return on assets (ROA) it did 40 years ago, the ROA for health care nearly doubled over the same period.
But to prosper with medical apps, the software makers need to do their homework - and maybe even change their culture.
Weight management vs. management of obesity
Not all medical-themed apps will fall under the FDA's purview. While a product marketed to help people lose weight might seem the same as a one designed to help treat obesity, to the FDA, it could mean the difference between giving the product a free pass and requiring extensive pre-release research.
As Thompson explained, an app advertised as helping people lose weight would probably count as a mere "wellness" program. It would therefore stay well outside the agency's remit. But if the product were marketed as a way to treat obesity - a disease - then it would be making a clinical claim and could fall under the agency's scrutiny.
"That's one of the things I'm preaching: these companies need to be aware of the rules, so when they make their claims, when they put their advertising together and other information that describes the use, they can make intelligent decisions about how they want to manage them," Thompson said.
Even if the product is regulated, not all regulations are equally tough.
If the medical app is substantially equivalent to one already out on the market (for example, two kinds of nearly identical image viewers), then the new product can go to market simply by proving substantial equivalence (strong similarities), Thompson explained. This is the 510 (k) process that the FDA rejected for Mobile MIM. But, as with Mobile MIM, if the FDA determines there is nothing already out there that's like it, the company will have to reclassify to create a designated category, or it'll need to navigate through a premarket approval application, which can be more difficult.
"The premarket approval application is a very resource-intensive process of doing clinical trials and proving the clinical safety effectiveness of their products," he said.
Culture clash
Software companies entering a regulated medical app field are also apt to face a culture clash. The Silicon Valley startup culture might be unaccustomed to the buttoned-up oversight culture of Bethesda.
"I think there's a cultural problem that the app makers are software companies unused to regulation," Thompson said. "They're using their old business model of quickly generating new software versions, trying to be first to the market, hoping that theirs will get all the brand recognition."
But for medical technologies, being first is sometimes a liability. The greater load of the regulatory burden often falls on the first one to make the product.
"The pioneer has a tougher row to hoe because they have to basically navigate where there's no map," Thompson said. "They have to figure the pathway through the agency and provide a certain amount of data and information to establish the right to market the product.
"Others can follow quickly on their heels, by proving they're just like the pioneer product. Oftentimes, that's an easier thing to do."
Since launching medical products requires deeper resource investment to address FDA regulations, the costs and effort involved do act as a barrier to entry. It can still pay to get to market first. It's likely competitors will still have to clear some hurdles, giving an opportunity for the pioneer to establish its product and be more likely to hold onto a top spot in that sector.
Crying wolf
However, not everyone thinks that the FDA's toughening stance is the problem. Regardless of what the FDA decides to do, some think the media's and industry's public panicking over the coming involvement could end up doing some of the greatest injury to the nascent business.
In a much talked-about post at his blog Creative Connectivity, British wireless technology guru Nick Hunn warned the industry that its "crying wolf" over coming FDA regulations will create more problems than it will solve.
"That degree of fear of the FDA regulations is putting established companies off from coming into that market," he told DOTmed. "It's causing concern for startups, and perhaps most worrying of all, it's making investors wary of putting money in a company if they have to go through a protracted approval process."
"[Investors] don't like things with a five-year approval cycle," he said.
Hunn worries that eventually U.S. companies, fearing FDA oversight, might stick to sports and fitness applications - largely unregulated by the agency - which means the United States will suffer as "most of the disruptive medical innovation will happen outside the United States," to the benefit of foreign startup companies.
This is bad not just for the bottomlines of would-be medical app entrepreneurs, but, Hunn thinks, for the system as a whole.
"There's a lot of concern that we're not going to see change in health care costs if we do it the way the medical community wants to do it. They do very well, thank you, out of the current system. We need something more disruptive," he said.
And, in fact, Hunn calls for a more decentralized approach to health care technology in the States as he hopes an alliance of software companies and patient advocacy groups can fight back against what he sees as the FDA's perhaps overweening authority.
"The FDA has really quite amazing powers. It can regulate almost anything to do with the health of anybody living in the U.S. and any pets and farm animals," he said. "But like most regulators, they move at an amazingly slow pace."
This slow pace, Hunn thinks, is applauded by traditional medical device manufacturers who benefit in some ways from the medical device regulatory environment that makes it hard for competitors to emerge.
"GE, Medtronic, are more than happy for regulation to stay the way it is," he said. "The best way to change it is to have network operators, consumer group manufacturers, coming in and sitting down as a body with the FDA to tell them, 'Look, the world is changing, technology is changing. You need to change so you can reflect and safely regulate the industry in the new form.'"
Hunn acknowledges that the FDA's main problem is that, as he puts it, "if they get something wrong, people will probably die. That's the risk and that's why they exist."
But he says its feeling that if it regulates strongly enough, it's not going to happen, might be short-sighted.
"In an ideal world, you could claim that's a good approach. But everything in life is actually a trade-off," he said.
"There's a degree of saying, the FDA probably needs to accept that in society going forward, patients need to take more responsibility for their own health care, and that means they're going to need to be allowed to use devices that might potentially do them harm," he said, much as they do with their (unregulated) diets.
When asked whether Hunn's ideas of a united front of business and patient groups lobbying for loosening FDA oversight would succeed, FDA law expert Thompson was cautious.
"Frankly, companies that have a collective interest in a given regulatory scheme would be wise to collaborate with one another on the regulatory environment," he said.
But he warned, "If their goal is simply to declare that their subject area is too important to regulate, too vital for the future of health care, I think their efforts are probably misplaced."
"The fact of the matter is, the FDA regulates an enormous amount of innovation. That's their job. That's what they do," he said.
He thinks the important question is not whether the technologies it is regulating have the potential to be truly innovative, but whether there is a "risk profile that necessitates" intervention.
And he thinks that in many ways the public likes what they perceive as the protective powers of the FDA.
"The challenge is where to draw the line," he said. "The question is, if something went wrong, would someone get hurt? That's the exact place where they tend to regulate, and society, frankly, wants them to regulate."
Radiology apps in practice
Aside from theoretical concerns, how have radiology apps actually worked in practice?
DOTmed News spoke with executives from Hartland, Wisc.-based Merge Healthcare, whose mobile eFilm application bowed at the Radiological Society of North America annual meeting nearly two years ago. Now available on the market, the app, which lets users check out radiology images, was never designed to be a diagnostic viewer, according to Merge. Feedback from radiologist and their customers after its debut made them take the path of turning it into what its promotional materials have dubbed a "workflow enhancement solution."
Part of Merge's concern is that radiology has, in the words of the blogger Hunn, some "particular issues."
"There have been instances in the past with some imaging, where facets of an image that was designed to be displayed on a high quality medical imaging system have been looked at on a computer screen, which has resulted in detail being missed because the resolutions were different, or compressions along the way had removed some of the artifacts," he said.
If it's not for diagnosing, what is it for?
To find out, Merge put DOTmed News in contact with a user of its eFilm Mobile.
"I find it really useful because you can kind of get a quick picture of what's going on," said Dr. Roman Klufas, a practicing radiologist. He is also president and medical director of Open MRI of New England and Advanced Radiology, Inc and staff neuroradiologist and instructor at Brigham and Women's Hospital in Boston.
Dr. Klufas said he typically uses the eFilm Mobile to gauge his workload - to see how many cases he has outstanding on the workstation - and he thinks in the future it can be used to share images with referring physicians. For instance, if a patient went to him under suspicion of having severe pneumonia, he can send scans of the lungs to the doctor, so the images can then be shared with the patient to break the good (or bad) news.
While Dr. Klufas didn't address this, others believe this could conceivably become a marketing strategy, something radiologists use to distinguish themselves from their rivals for referring physicians - instead of having to wait to get a grainy fax to show your anxious patient, come to us, and we'll give you instant clear, full-color pictures on your iPhone to ease worried minds.
Gray areas
Another workflow use Dr. Klufas pointed out is advising the technologist operating the machine based on the images he is seeing.
Tim Kulbago, chief technology officer at Merge, described the scenario thusly: a radiologist is driving home at the end of the day. The sonographer performing the ultrasound - and the quality of the image is based on the technologists' skill, Kulbago said - sends an image of the scan to the radiologist's smartphone, asking if the image is clear enough for the patient to go home, or if another scan is needed.
"[The radiologist is] not reading the study, but simply making the decision as to whether the patient can go home or not, and having some level of confidence that you won't have to bring the patient back," Kulbago said.
Kulbago thinks this is important because it improves workflow; it meets a real need and is not mere mobile gimcrackery.
"You've got a couple of companies out there that say, 'Oh, look, you can see your study.' But if it's really that important, you can find a workstation to see it. [These apps are] not a read-at-home story," he said.
Nonetheless, even the suggested use brings us into murky waters. Deciding whether a study is good enough to keep for later is still something of an act of medical interpretation, even if no actual diagnosis is made off the viewer. Even more so is the radiologist-on-the-golf-course example. A spokesperson from Merge and representatives from other companies used the analogy of the clinic or perhaps a puzzled colleague sending an image to a radiologist on the golf course to ask for advice or an opinion.
"Can you take a look? Is it diagnostic? Is it a gray area?" asked Kulbago. "Yeah, it's a gray area."
"It's kind of the same thing with the ultrasound," he said. "Is there some kind of medical [act] going on? For sure. Is it an appropriate use of the technology to prevent patient callbacks, patient frustrations and lower costs and improve health care? It probably is."
Comparable clarity?
Dr. Klufas said that while he has no data to support his observations, in his practice he has checked chest X-rays on his workstation and on the eFilm Mobile viewer and wasn't able to see a big difference.
"The images are definitely comparable," he said, "though I would assume that the matrix is not quite obviously the same as it's going to be on a regular diagnostic workstation."
He suspects that for MRI and CT scan images, but perhaps not for film, it will, one day, be diagnostically useful.
While clinical evidence is at the early stages, a study conducted earlier this year and published in the American Journal of Roentgenology found that radiologists making (test) diagnoses on software on an iPhone and a PDA did about the same, if not slightly better, than when working on other secondary class (non-diagnostic quality) monitors.
Clinical diagnosing in Canada
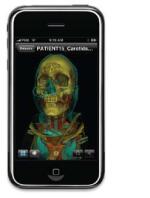
In any case, the age of mobile diagnoses is already upon us, at least across the 49th parallel. In April, Canada Health, our northern neighbor's version of the FDA, OK'd radiologists making clinical diagnoses off Calgary Scientific's ResolutionMD, the company said.
Using PureWeb - a "zero footprint" product - the app basically runs off a web browser on the iPhone, iPad or Android, and lets the mobile device, in essence, "tap" into a workstation. Unlike most viewers, the app itself is hosting none of the software, a fact Byron Osing, CEO and chair of Calgary Scientific, credits with its approval by Canada Health. (Though made by Calgary, it is distributed in Canada through Belgium-based film and software giant Agfa, Osing said.)
"Most of the applications filed by companies to the FDA that have been outright declined have been declined because the software runs resident on the device itself," Osing said. "In almost all the cases the data files have to be pushed out to the device for processing. In that case, the company would then have to prove that the device itself had the processing power and memory to be a, 'de facto PACs or advanced workstation'," he said.
"That makes it difficult for anybody with that kind of architecture because handheld devices don't have that kind of memory."
Clinical studies
Calgary Scientific has run three major clinical studies at two research hospitals in the United States and one in Canada, Osing said. Although because of a non-disclosure agreement with the hospitals, which are looking to publish the work in scientific journals, he declined to say which ones.
He said the studies uncovered a near-perfect correlation in the ability to diagnose on the handheld and a monitor; a couple of the studies even showed there was a 25 percent better visual capability in terms of clarity and pixelation on handhelds than older PACS workstations.
And the main use, according to Osing: the stroke scenario. While many hospitals have access to CT scanners, a lot don't have round-the-clock specialists. Patients entering the emergency room showing acute stroke symptoms might have to be packed into a helicopter and sent to a hospital where their condition could be diagnosed and clot-busting agents delivered to save their lives and preserve brain function. But with his company's app, a scan could be transmitted immediately to an on-call radiologist - wherever he is, he could be at home - delivering a notification to come and do a full diagnosis off of the image that came from the scanner.
"What it does in essence, it saves lives, it saves long-term neurological damage and huge costs to the medical system," Osing said. "Instead of a medical bill of a few thousands dollars - if you have long-term neurological damage, you're in the hundreds of thousands of dollars of health care costs."
But it remains to be seen if the FDA agrees. Calgary Scientific has already begun the application process. "They're now studying...our application very carefully," Osing said. "We're in the wait-and-see-how-it-goes [phase]."
The company is not alone in launching clinical trials to provide real, hard data to replace what's currently mostly speculation. Two months ago, Merge started a pilot project with Massachusetts General Hospital to determine where imaging mobile can assist in the clinical workflow. The company is also looking eagerly at the bigger viewing real estate offered by that much-hyped-for-health-care device: the iPad.
"It's going to be really interesting," said Merge's Kulbago about what he considers the still largely open future for mobile in medicine. "It's kind of the story of the microwave oven: nobody had the desire to cook a hotdog in 30 seconds before the microwave came along. Now, why would you wait more than 30 seconds to cook a hotdog?"
About a year and a half ago, the U.S. Food and Drug Administration made a decision that many in the booming smartphone medical app industry regard as menacing.
Shortly after the Apple Store launched, the agency told Apple to remove an image viewer produced by the Cleveland-based MIMVista from its virtual shelves. The viewer, Mobile MIM, earned the dubious distinction of being among the first radiology iPhone apps ever taken out of circulation by the agency.
In January, the FDA did something to disturb industry-watchers even more: it rejected the second of MIMVista's so-called fast-track, or 510 (k), applications, requiring the company to go through the lengthy, and expensive, premarket approval before the product could ever be sold by the Apple Store.
Of course, this was an isolated case. Mostly, smartphone users with a medical bent have been in clover. According to a recent MobiHealthNews industry survey, there are around 5,820 medical, health or fitness-themed apps for devices such as the iPhone, iPod Touch and the various Google-run Android platforms. These apps range from iTriage, which lets users check wait times at nearby hospital emergency rooms, to Epocrates, an incredibly popular guide for drug interactions for doctors, and the sundry "wellness" apps that help people schedule workouts and plan their diets. Surveys have shown between two-thirds to three-quarters of doctors own and use some sort of smartphone and often carry the device on rounds.
Yet the news of Mobile MIM's plight coupled with signs of a tougher FDA regulatory attitude compels many to wonder whether they face a brewing storm: will the FDA soon clamp down on the thousands of health care smartphone apps? Will the agency focus mostly on radiology viewers - where there is clear potential for dangerous misreadings by rushed radiologists on tiny screens - or is just the fear of coming regulations going to, in the end, do the most harm?
Light touch
After the initial pulling of the Mobile MIM, few app companies announced being contacted by the FDA, according to most reports. In fact, regulations have actually been fairly light, observed Bradley Merrill Thompson, partner at Epstein Becker and Green, P.C. in Washington D.C. and counsel for EBG Advisors.
"I think the FDA, to their credit, has been holding back to study what's going on, to try to understand the hardware and software and decide what they want to do based on risk," Thompson said.
An authority on medical device law, Thompson literally wrote the book on the subject, "FDA Regulation of Medical Devices," published by Interpharm Press in 1995. He has also testified before Congress on FDA rulemaking.
Planning ahead
Nonetheless, the FDA is taking a closer look at the industry, Thompson said. But even with tougher scrutiny, he thinks the field will remain extremely attractive to companies. Simply put, health care is a good place to be, even with - or in some cases due to - regulatory hassles.
In one of a series of articles published in MobiHealthNews about FDA regulations of smartphone apps, Thompson cited a Thomson Reuters study that showed medical device makers enjoying an average of five-year gross margins of 59 percent, compared with about 46 percent for the Standard & Poor 500. And a Deloitte Center for the Edge study also cited by Thompson found that unlike most of the economy, which only provides one quarter of the return on assets (ROA) it did 40 years ago, the ROA for health care nearly doubled over the same period.
But to prosper with medical apps, the software makers need to do their homework - and maybe even change their culture.
Weight management vs. management of obesity
Not all medical-themed apps will fall under the FDA's purview. While a product marketed to help people lose weight might seem the same as a one designed to help treat obesity, to the FDA, it could mean the difference between giving the product a free pass and requiring extensive pre-release research.
As Thompson explained, an app advertised as helping people lose weight would probably count as a mere "wellness" program. It would therefore stay well outside the agency's remit. But if the product were marketed as a way to treat obesity - a disease - then it would be making a clinical claim and could fall under the agency's scrutiny.
"That's one of the things I'm preaching: these companies need to be aware of the rules, so when they make their claims, when they put their advertising together and other information that describes the use, they can make intelligent decisions about how they want to manage them," Thompson said.
Even if the product is regulated, not all regulations are equally tough.
If the medical app is substantially equivalent to one already out on the market (for example, two kinds of nearly identical image viewers), then the new product can go to market simply by proving substantial equivalence (strong similarities), Thompson explained. This is the 510 (k) process that the FDA rejected for Mobile MIM. But, as with Mobile MIM, if the FDA determines there is nothing already out there that's like it, the company will have to reclassify to create a designated category, or it'll need to navigate through a premarket approval application, which can be more difficult.
"The premarket approval application is a very resource-intensive process of doing clinical trials and proving the clinical safety effectiveness of their products," he said.
Culture clash
Software companies entering a regulated medical app field are also apt to face a culture clash. The Silicon Valley startup culture might be unaccustomed to the buttoned-up oversight culture of Bethesda.
"I think there's a cultural problem that the app makers are software companies unused to regulation," Thompson said. "They're using their old business model of quickly generating new software versions, trying to be first to the market, hoping that theirs will get all the brand recognition."
But for medical technologies, being first is sometimes a liability. The greater load of the regulatory burden often falls on the first one to make the product.
"The pioneer has a tougher row to hoe because they have to basically navigate where there's no map," Thompson said. "They have to figure the pathway through the agency and provide a certain amount of data and information to establish the right to market the product.
"Others can follow quickly on their heels, by proving they're just like the pioneer product. Oftentimes, that's an easier thing to do."
Since launching medical products requires deeper resource investment to address FDA regulations, the costs and effort involved do act as a barrier to entry. It can still pay to get to market first. It's likely competitors will still have to clear some hurdles, giving an opportunity for the pioneer to establish its product and be more likely to hold onto a top spot in that sector.
Crying wolf
However, not everyone thinks that the FDA's toughening stance is the problem. Regardless of what the FDA decides to do, some think the media's and industry's public panicking over the coming involvement could end up doing some of the greatest injury to the nascent business.
In a much talked-about post at his blog Creative Connectivity, British wireless technology guru Nick Hunn warned the industry that its "crying wolf" over coming FDA regulations will create more problems than it will solve.
"That degree of fear of the FDA regulations is putting established companies off from coming into that market," he told DOTmed. "It's causing concern for startups, and perhaps most worrying of all, it's making investors wary of putting money in a company if they have to go through a protracted approval process."
"[Investors] don't like things with a five-year approval cycle," he said.
Hunn worries that eventually U.S. companies, fearing FDA oversight, might stick to sports and fitness applications - largely unregulated by the agency - which means the United States will suffer as "most of the disruptive medical innovation will happen outside the United States," to the benefit of foreign startup companies.
This is bad not just for the bottomlines of would-be medical app entrepreneurs, but, Hunn thinks, for the system as a whole.
"There's a lot of concern that we're not going to see change in health care costs if we do it the way the medical community wants to do it. They do very well, thank you, out of the current system. We need something more disruptive," he said.
And, in fact, Hunn calls for a more decentralized approach to health care technology in the States as he hopes an alliance of software companies and patient advocacy groups can fight back against what he sees as the FDA's perhaps overweening authority.
"The FDA has really quite amazing powers. It can regulate almost anything to do with the health of anybody living in the U.S. and any pets and farm animals," he said. "But like most regulators, they move at an amazingly slow pace."
This slow pace, Hunn thinks, is applauded by traditional medical device manufacturers who benefit in some ways from the medical device regulatory environment that makes it hard for competitors to emerge.
"GE, Medtronic, are more than happy for regulation to stay the way it is," he said. "The best way to change it is to have network operators, consumer group manufacturers, coming in and sitting down as a body with the FDA to tell them, 'Look, the world is changing, technology is changing. You need to change so you can reflect and safely regulate the industry in the new form.'"
Hunn acknowledges that the FDA's main problem is that, as he puts it, "if they get something wrong, people will probably die. That's the risk and that's why they exist."
But he says its feeling that if it regulates strongly enough, it's not going to happen, might be short-sighted.
"In an ideal world, you could claim that's a good approach. But everything in life is actually a trade-off," he said.
"There's a degree of saying, the FDA probably needs to accept that in society going forward, patients need to take more responsibility for their own health care, and that means they're going to need to be allowed to use devices that might potentially do them harm," he said, much as they do with their (unregulated) diets.
When asked whether Hunn's ideas of a united front of business and patient groups lobbying for loosening FDA oversight would succeed, FDA law expert Thompson was cautious.
"Frankly, companies that have a collective interest in a given regulatory scheme would be wise to collaborate with one another on the regulatory environment," he said.
But he warned, "If their goal is simply to declare that their subject area is too important to regulate, too vital for the future of health care, I think their efforts are probably misplaced."
"The fact of the matter is, the FDA regulates an enormous amount of innovation. That's their job. That's what they do," he said.
He thinks the important question is not whether the technologies it is regulating have the potential to be truly innovative, but whether there is a "risk profile that necessitates" intervention.
And he thinks that in many ways the public likes what they perceive as the protective powers of the FDA.
"The challenge is where to draw the line," he said. "The question is, if something went wrong, would someone get hurt? That's the exact place where they tend to regulate, and society, frankly, wants them to regulate."
Radiology apps in practice
Aside from theoretical concerns, how have radiology apps actually worked in practice?
DOTmed News spoke with executives from Hartland, Wisc.-based Merge Healthcare, whose mobile eFilm application bowed at the Radiological Society of North America annual meeting nearly two years ago. Now available on the market, the app, which lets users check out radiology images, was never designed to be a diagnostic viewer, according to Merge. Feedback from radiologist and their customers after its debut made them take the path of turning it into what its promotional materials have dubbed a "workflow enhancement solution."
Part of Merge's concern is that radiology has, in the words of the blogger Hunn, some "particular issues."
"There have been instances in the past with some imaging, where facets of an image that was designed to be displayed on a high quality medical imaging system have been looked at on a computer screen, which has resulted in detail being missed because the resolutions were different, or compressions along the way had removed some of the artifacts," he said.
If it's not for diagnosing, what is it for?
To find out, Merge put DOTmed News in contact with a user of its eFilm Mobile.
"I find it really useful because you can kind of get a quick picture of what's going on," said Dr. Roman Klufas, a practicing radiologist. He is also president and medical director of Open MRI of New England and Advanced Radiology, Inc and staff neuroradiologist and instructor at Brigham and Women's Hospital in Boston.
Dr. Klufas said he typically uses the eFilm Mobile to gauge his workload - to see how many cases he has outstanding on the workstation - and he thinks in the future it can be used to share images with referring physicians. For instance, if a patient went to him under suspicion of having severe pneumonia, he can send scans of the lungs to the doctor, so the images can then be shared with the patient to break the good (or bad) news.
While Dr. Klufas didn't address this, others believe this could conceivably become a marketing strategy, something radiologists use to distinguish themselves from their rivals for referring physicians - instead of having to wait to get a grainy fax to show your anxious patient, come to us, and we'll give you instant clear, full-color pictures on your iPhone to ease worried minds.
Gray areas
Another workflow use Dr. Klufas pointed out is advising the technologist operating the machine based on the images he is seeing.
Tim Kulbago, chief technology officer at Merge, described the scenario thusly: a radiologist is driving home at the end of the day. The sonographer performing the ultrasound - and the quality of the image is based on the technologists' skill, Kulbago said - sends an image of the scan to the radiologist's smartphone, asking if the image is clear enough for the patient to go home, or if another scan is needed.
"[The radiologist is] not reading the study, but simply making the decision as to whether the patient can go home or not, and having some level of confidence that you won't have to bring the patient back," Kulbago said.
Kulbago thinks this is important because it improves workflow; it meets a real need and is not mere mobile gimcrackery.
"You've got a couple of companies out there that say, 'Oh, look, you can see your study.' But if it's really that important, you can find a workstation to see it. [These apps are] not a read-at-home story," he said.
Nonetheless, even the suggested use brings us into murky waters. Deciding whether a study is good enough to keep for later is still something of an act of medical interpretation, even if no actual diagnosis is made off the viewer. Even more so is the radiologist-on-the-golf-course example. A spokesperson from Merge and representatives from other companies used the analogy of the clinic or perhaps a puzzled colleague sending an image to a radiologist on the golf course to ask for advice or an opinion.
"Can you take a look? Is it diagnostic? Is it a gray area?" asked Kulbago. "Yeah, it's a gray area."
"It's kind of the same thing with the ultrasound," he said. "Is there some kind of medical [act] going on? For sure. Is it an appropriate use of the technology to prevent patient callbacks, patient frustrations and lower costs and improve health care? It probably is."
Comparable clarity?
Dr. Klufas said that while he has no data to support his observations, in his practice he has checked chest X-rays on his workstation and on the eFilm Mobile viewer and wasn't able to see a big difference.
"The images are definitely comparable," he said, "though I would assume that the matrix is not quite obviously the same as it's going to be on a regular diagnostic workstation."
He suspects that for MRI and CT scan images, but perhaps not for film, it will, one day, be diagnostically useful.
While clinical evidence is at the early stages, a study conducted earlier this year and published in the American Journal of Roentgenology found that radiologists making (test) diagnoses on software on an iPhone and a PDA did about the same, if not slightly better, than when working on other secondary class (non-diagnostic quality) monitors.
Clinical diagnosing in Canada
In any case, the age of mobile diagnoses is already upon us, at least across the 49th parallel. In April, Canada Health, our northern neighbor's version of the FDA, OK'd radiologists making clinical diagnoses off Calgary Scientific's ResolutionMD, the company said.
Using PureWeb - a "zero footprint" product - the app basically runs off a web browser on the iPhone, iPad or Android, and lets the mobile device, in essence, "tap" into a workstation. Unlike most viewers, the app itself is hosting none of the software, a fact Byron Osing, CEO and chair of Calgary Scientific, credits with its approval by Canada Health. (Though made by Calgary, it is distributed in Canada through Belgium-based film and software giant Agfa, Osing said.)
"Most of the applications filed by companies to the FDA that have been outright declined have been declined because the software runs resident on the device itself," Osing said. "In almost all the cases the data files have to be pushed out to the device for processing. In that case, the company would then have to prove that the device itself had the processing power and memory to be a, 'de facto PACs or advanced workstation'," he said.
"That makes it difficult for anybody with that kind of architecture because handheld devices don't have that kind of memory."
Clinical studies
Calgary Scientific has run three major clinical studies at two research hospitals in the United States and one in Canada, Osing said. Although because of a non-disclosure agreement with the hospitals, which are looking to publish the work in scientific journals, he declined to say which ones.
He said the studies uncovered a near-perfect correlation in the ability to diagnose on the handheld and a monitor; a couple of the studies even showed there was a 25 percent better visual capability in terms of clarity and pixelation on handhelds than older PACS workstations.
And the main use, according to Osing: the stroke scenario. While many hospitals have access to CT scanners, a lot don't have round-the-clock specialists. Patients entering the emergency room showing acute stroke symptoms might have to be packed into a helicopter and sent to a hospital where their condition could be diagnosed and clot-busting agents delivered to save their lives and preserve brain function. But with his company's app, a scan could be transmitted immediately to an on-call radiologist - wherever he is, he could be at home - delivering a notification to come and do a full diagnosis off of the image that came from the scanner.
"What it does in essence, it saves lives, it saves long-term neurological damage and huge costs to the medical system," Osing said. "Instead of a medical bill of a few thousands dollars - if you have long-term neurological damage, you're in the hundreds of thousands of dollars of health care costs."
But it remains to be seen if the FDA agrees. Calgary Scientific has already begun the application process. "They're now studying...our application very carefully," Osing said. "We're in the wait-and-see-how-it-goes [phase]."
The company is not alone in launching clinical trials to provide real, hard data to replace what's currently mostly speculation. Two months ago, Merge started a pilot project with Massachusetts General Hospital to determine where imaging mobile can assist in the clinical workflow. The company is also looking eagerly at the bigger viewing real estate offered by that much-hyped-for-health-care device: the iPad.
"It's going to be really interesting," said Merge's Kulbago about what he considers the still largely open future for mobile in medicine. "It's kind of the story of the microwave oven: nobody had the desire to cook a hotdog in 30 seconds before the microwave came along. Now, why would you wait more than 30 seconds to cook a hotdog?"