by
John R. Fischer, Senior Reporter | February 10, 2020
Phase III clinical trials have been set in motion for a set of fluorescent markers used for targeting cancer during surgery, following positive findings for their use in a multi-institutional Phase II clinical trial.
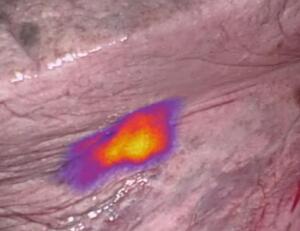
Researchers at On Target Laboratories found that the marker treatment — called OTL38 — helped improve outcomes for 26 percent of patients who underwent pulmonary resection for non-small-cell lung cancer (NSCLC).
"OTL38 is the first technique that is specific to imaging adenocarcinomas of the lung, which is one of the most common types of invasive lung cancer," said Dr. Inderpal (Netu) S. Sarkaria of the Department of Cardiothoracic Surgery at the University of Pittsburgh Medical Center, in a statement. "Near-infrared imaging with OTL38 may be a powerful tool to help surgeons significantly improve the quality of lung cancer surgery by more clearly identifying tumors and allowing the surgeon to better see and completely remove them — one of the most vital components in the overall care of patients with this disease."



Ad Statistics
Times Displayed: 365450
Times Visited: 7046 Quality remanufactured Certified Centrifuges at Great prices! Fully warranted and backed by a company you can trust! Call or click for a free quote today! www.Centrifugestore.com 800-457-7576
Developed at the Purdue laboratory of Philip Low in the Purdue Institute of Drug Discovery, the treatment is a form of intraoperative molecular imaging (also known as fluorescence-guided surgery or IMI) and may increase a person’s chances of receiving a complete surgical resection, raising their likelihood of survival and reducing re-operations or adjuvant treatment for hospitals.
The marker was applied to 92 patients with operable stage I-II non-small-cell lung cancer over 18 months. Such patients are often treated with pulmonary resection, a procedure that is performed with either a wedge resection, segmentectomy, or lobectomy. No drug-related serious adverse events were reported, and 24 patients said they felt an impact, with eight percent experiencing a change in their stage due to the use of the IMI.
It also improved localization of small and peripheral lesions, which are difficult for surgeons to identify visually or through manual palpitation, and enabled localization of otherwise unlocalizable lesions in 11 patients. Another 10 cancers were found in seven patients, and while surgeons during the Specimen Check Phase thought all margins were adequate, back-table inspection using IMI revealed inadequate margins in eight patients.
"Our goal is to provide surgeons with new technology to help them provide a more complete resection to more patients. This gives patients the best chance of improved outcomes after surgery," said Christopher Barys, president and CEO of On Target Laboratories, in a statement.
OTL38 is currently under development in two Phase III clinical trials for lung and ovarian cancer indications, and has received a fast-track designation for both, as well as an orphan designation for ovarian cancer from the FDA. Both are being carried out under a special protocol agreement with the FDA.
The findings of the study were presented in January at the 56th annual meeting of the Society of Thoracic Surgeons in New Orleans.