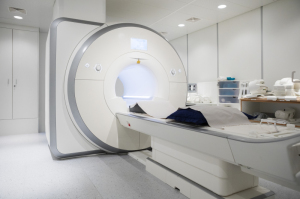
The cloud is expected to provide infinite
archiving and on-demand computing power
as required for deep learning, AI & other
applications used in MR.
Picture: Siemens Prisma 3T system at
Columbia Magnetic Resonance Research Center
archiving and on-demand computing power
as required for deep learning, AI & other
applications used in MR.
Picture: Siemens Prisma 3T system at
Columbia Magnetic Resonance Research Center
Implants, gadolinium and AI: Changing perceptions in MR
November 26, 2018
by John R. Fischer, Senior Reporter
A patient undergoing an MR scan may soon be in and out in a fraction of the time, thanks to a new partnership between the department of radiology at NYU School of Medicine and ubiquitous social media giant Facebook.
The two are pooling their resources into a new artificial intelligence project called FastMRI, with the goal of making MR scans ten times faster without compromising image quality.
“This particular collaboration starts at the beginning of imaging MR and has to do with image acquisition and reconstruction of the image,” Dr. Yvonne Lui, a neuroradiologist and associate chair of artificial intelligence in the department of radiology at NYU School of Medicine who is heading the project, told HCB News. “The focus is on how we can acquire less data in order to make the same quality diagnostic image.”
Like Lui, many radiologists and other clinicians are investigating the potential uses of AI and machine learning for MR scans, with applications ranging from tumor segmentation to lesion detection, to image classification.
But AI is merely one emerging area of interest within the MR world, with each poised to have its own impact on the ways in which scanners are used, the quality of care delivered to patients and the affordability of exams.
AI and the cloud
A typical MR scan ranges from 15 minutes to over an hour and requires patients to remain very still and hold their breath for lengths of time in narrow bores when imaging certain regions of the body. For many, particularly children, these conditions are uncomfortable, and can cause anxiety or claustrophobia.
While Lui and her colleagues hope to use machine learning to reduce these experiences by decreasing scan time, she says right now there is also a lot of interest in lesion detection and image classification.
“Is there cancer or no cancer? Is there a bleed or no bleed? Is there something abnormal and if so, where is it and can you put a box around it?” she asked. “Detecting and interpreting images is where a lot of the interest lies.”
FastMRI is hardly the only artificial intelligence initiative taking aim at MR, but all of these endeavors – regardless of what their individual goals may be – share a fundamental need.
With any of these applications comes the need for scalable infinite memory, infinite archive capacity and on-demand infinite computing power, all of which can be found in the cloud.
“It’s the only way to combine these bigger bodies of data into one, large coordinated resource that allows us to operate together more efficiently,” said Dr. John Thomas Vaughan, Jr., a professor and director of the Columbia MR Research Center at Columbia University.
The Center recently became the first cloud-based MR research center with the deployment this summer of its Flywheel data management suite on to the Google Cloud. In addition to storing huge amounts of data, the initiative enables researchers working at different sites on campus as well as those at partnership institutions to access data obtained from one another without the need for manual transport, and other slower, less secure means, yielding more efficient research and collaboration.
Though in the beginning stages, the Center has already attracted interest from institutions in the U.S., China and India wishing to replicate the model. Vaughan says he perceives similar initiatives around the cloud arising in the future and bringing greater access to necessary biomedical diagnostics and therapies worldwide.
“MR has only been delivered to 10 percent of the world’s population, says the World Health Organization,” said Vaughan. “With the cloud, we can reach the rest of the world quite literally to provide access to MR as well as other very safe and very powerful tools in medicine and science. Our cloud-based center facilitates less redundancy of equipment and staff for maintenance and support. It’s also a nice way to have a distributed lab around the world. I can run a lab right here from my desk with the power of the cloud.”
Questions regarding gadolinium fuel innovation
Much discussion over the last few years has surrounded the use of gadolinium, the workhorse of MR contrast agents. For over a decade, the scientific community has known that gadolinium brings certain risks to patients with renal insufficiencies, but more recent evidence that the agent can accumulate in the brain has left many experts and regulators questioning its reputation as a safe source of contrast.
One high profile case was the lawsuit brought by action star Chuck Norris, and his wife, Gena, against 11 drug companies last November, alleging that the injection of gadolinium-based contrast agents (GBCAs) in Gena during a series of MR scans led her to develop gadolinium deposition disease and cost the couple more than $2 million in out-of-pocket costs for hospitalizations and treatments.
To date, researchers have been unable to find any evidence of neurological damage or other adverse effects relating to the found gadolinium retention in the brain, but there is a renewed effort in the industry to investigate the issue. Meanwhile, some researchers are investigating alternative methods for creating readable MR scans using alternative contrast agents or no agents at all.
A recent Ph.D. graduate from Stanford, Enhao Gong and Dr. Greg Zaharchuk from Radiology at Stanford, founded a startup called Subtle Medical Inc., which is developing an AI solution from technology validated at Stanford that aims to cut the gadolinium dose to 10 percent during MR exams.
“We are getting more transparent about gadolinium, and whether or not we want to use it,” said Dr. Gong. “With AI, you can enhance the quality of the image so that 10 percent dosage is the same as 100 percent dosage. We can train software to read images better than the human eye, so that means we need less contrast.”
Changes are also taking place on regulatory levels, with the FDA recently issuing a requirement for manufacturers to include gadolinium retention label warnings for GBCAs to relay information on gadolinium remaining in the bodies of patients, including the brain, for months to years after.
The decision followed an extensive review and talks with the Medical Imaging Drugs Advisory Committee, though no adverse health impacts were found in its investigation. In addition, the agency will also require manufacturers to conduct additional studies on animals and will conduct a study on patients who undergo repeat GBCA MR screenings to investigate the possibility of neurologic effects.
A new perspective on implants and MR
Like gadolinium, safety concerns have also surrounded the compatibility of implants in MR environments, with many radiologists hesitant to perform scans on patients with implants lacking MR-conditional labeling. While proven relatively safe in numerous studies, it was not until the decision this spring by the Centers for Medicare and Medicaid Services to cover such devices, that many began to warm up to the idea.
“When MR conditional devices that were labeled by the FDA first came out, they were tested and designed to indicate that under certain specified conditions, they would be safe when subjected to MR so that patients with those devices could be scanned,” said Dr. Henry Halperin, professor of medicine, radiology and biomedical engineering at Johns Hopkins University. “For devices that were not specifically designed to be MR-conditional, the presumption was that that they were not safe, although there were small amounts of data to suggest that it was okay.”
A study completed by Halperin at the end of last year found that such safety extended to “legacy” pacemakers and defibrillators, finding that anyone implanted with these devices, under few restrictions, could undergo an MR scan without incurring adverse effects.
This, along with other findings, led CMS in April to expand coverage to implanted pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy pacemakers, and cardiac resynchronization therapy defibrillators.
Halperin says that the expansion opens up greater access to necessary procedures for patients that were once deemed high-risk for MR exams due to their implants, and that the change has been received well within the cardiology community. Such a switch, though, will take a while for radiologists to come around to, he warns.
“When radiologists were trained years ago, they were taught that it was unsafe and dangerous to do MR scans in patients with pacemakers and inflammable defibrillators. Now, they have to kind of ‘unlearn’ that or evolve with the current thinking,” he said. “They just need to be made more aware of the current literature. This data has been published only in the last year, and it may not be disseminated to people so quickly. I think the radiologists are little bit behind on that but slowly catching up.”
The bottom line
Increasing coverage of implants, along with AI, the cloud and gadolinium reduction are all expected to act as drivers in the increasing utilization of MR, along with increases in labor, consumable costs and services. The advent of new technologies such as higher strength magnets, nanotechnologies, and wide-bore and lighter-weighing systems will also make their mark, helping to drive the MR market from its current value of $5.8 billion at a rate of 3.9 percent annually.
“MR will always be a costly test,” said James Laskaris, a clinical strategist for MD Buyline. “But the key is it will be a cost avoidance technology by either ruling out or diagnosing a disease earlier and more accurately.”
Echoing this sentiment is Dr. Sriram Mannava, quality improvement and safety officer at Columbus Radiology Corporation, a Radiology Partners practice, who perceives utilization continuing to increase outside of outpatient settings and in ways in which MR is applied to those who require it.
“There is a really big push by payors right now for high-quality, low-cost imaging, and we’re seeing a lot less of hospital outpatient imaging because it costs so much more,” he said. “MR is not an inexpensive modality, but the information we get from it can be very useful. What we need to make sure of is that we are using it in an appropriate fashion.”
The two are pooling their resources into a new artificial intelligence project called FastMRI, with the goal of making MR scans ten times faster without compromising image quality.
“This particular collaboration starts at the beginning of imaging MR and has to do with image acquisition and reconstruction of the image,” Dr. Yvonne Lui, a neuroradiologist and associate chair of artificial intelligence in the department of radiology at NYU School of Medicine who is heading the project, told HCB News. “The focus is on how we can acquire less data in order to make the same quality diagnostic image.”
Like Lui, many radiologists and other clinicians are investigating the potential uses of AI and machine learning for MR scans, with applications ranging from tumor segmentation to lesion detection, to image classification.
But AI is merely one emerging area of interest within the MR world, with each poised to have its own impact on the ways in which scanners are used, the quality of care delivered to patients and the affordability of exams.
AI and the cloud
A typical MR scan ranges from 15 minutes to over an hour and requires patients to remain very still and hold their breath for lengths of time in narrow bores when imaging certain regions of the body. For many, particularly children, these conditions are uncomfortable, and can cause anxiety or claustrophobia.
While Lui and her colleagues hope to use machine learning to reduce these experiences by decreasing scan time, she says right now there is also a lot of interest in lesion detection and image classification.
“Is there cancer or no cancer? Is there a bleed or no bleed? Is there something abnormal and if so, where is it and can you put a box around it?” she asked. “Detecting and interpreting images is where a lot of the interest lies.”
FastMRI is hardly the only artificial intelligence initiative taking aim at MR, but all of these endeavors – regardless of what their individual goals may be – share a fundamental need.
With any of these applications comes the need for scalable infinite memory, infinite archive capacity and on-demand infinite computing power, all of which can be found in the cloud.
“It’s the only way to combine these bigger bodies of data into one, large coordinated resource that allows us to operate together more efficiently,” said Dr. John Thomas Vaughan, Jr., a professor and director of the Columbia MR Research Center at Columbia University.
The Center recently became the first cloud-based MR research center with the deployment this summer of its Flywheel data management suite on to the Google Cloud. In addition to storing huge amounts of data, the initiative enables researchers working at different sites on campus as well as those at partnership institutions to access data obtained from one another without the need for manual transport, and other slower, less secure means, yielding more efficient research and collaboration.
Though in the beginning stages, the Center has already attracted interest from institutions in the U.S., China and India wishing to replicate the model. Vaughan says he perceives similar initiatives around the cloud arising in the future and bringing greater access to necessary biomedical diagnostics and therapies worldwide.
Much interest surrounding AI's role in MR is focused
on image detection and interpretation, as well as
tumor segmentation and speeding up scans.
on image detection and interpretation, as well as
tumor segmentation and speeding up scans.
Questions regarding gadolinium fuel innovation
Much discussion over the last few years has surrounded the use of gadolinium, the workhorse of MR contrast agents. For over a decade, the scientific community has known that gadolinium brings certain risks to patients with renal insufficiencies, but more recent evidence that the agent can accumulate in the brain has left many experts and regulators questioning its reputation as a safe source of contrast.
One high profile case was the lawsuit brought by action star Chuck Norris, and his wife, Gena, against 11 drug companies last November, alleging that the injection of gadolinium-based contrast agents (GBCAs) in Gena during a series of MR scans led her to develop gadolinium deposition disease and cost the couple more than $2 million in out-of-pocket costs for hospitalizations and treatments.
To date, researchers have been unable to find any evidence of neurological damage or other adverse effects relating to the found gadolinium retention in the brain, but there is a renewed effort in the industry to investigate the issue. Meanwhile, some researchers are investigating alternative methods for creating readable MR scans using alternative contrast agents or no agents at all.
A recent Ph.D. graduate from Stanford, Enhao Gong and Dr. Greg Zaharchuk from Radiology at Stanford, founded a startup called Subtle Medical Inc., which is developing an AI solution from technology validated at Stanford that aims to cut the gadolinium dose to 10 percent during MR exams.
“We are getting more transparent about gadolinium, and whether or not we want to use it,” said Dr. Gong. “With AI, you can enhance the quality of the image so that 10 percent dosage is the same as 100 percent dosage. We can train software to read images better than the human eye, so that means we need less contrast.”
Changes are also taking place on regulatory levels, with the FDA recently issuing a requirement for manufacturers to include gadolinium retention label warnings for GBCAs to relay information on gadolinium remaining in the bodies of patients, including the brain, for months to years after.
The decision followed an extensive review and talks with the Medical Imaging Drugs Advisory Committee, though no adverse health impacts were found in its investigation. In addition, the agency will also require manufacturers to conduct additional studies on animals and will conduct a study on patients who undergo repeat GBCA MR screenings to investigate the possibility of neurologic effects.
A new perspective on implants and MR
Like gadolinium, safety concerns have also surrounded the compatibility of implants in MR environments, with many radiologists hesitant to perform scans on patients with implants lacking MR-conditional labeling. While proven relatively safe in numerous studies, it was not until the decision this spring by the Centers for Medicare and Medicaid Services to cover such devices, that many began to warm up to the idea.
“When MR conditional devices that were labeled by the FDA first came out, they were tested and designed to indicate that under certain specified conditions, they would be safe when subjected to MR so that patients with those devices could be scanned,” said Dr. Henry Halperin, professor of medicine, radiology and biomedical engineering at Johns Hopkins University. “For devices that were not specifically designed to be MR-conditional, the presumption was that that they were not safe, although there were small amounts of data to suggest that it was okay.”
A study completed by Halperin at the end of last year found that such safety extended to “legacy” pacemakers and defibrillators, finding that anyone implanted with these devices, under few restrictions, could undergo an MR scan without incurring adverse effects.
This, along with other findings, led CMS in April to expand coverage to implanted pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy pacemakers, and cardiac resynchronization therapy defibrillators.
Halperin says that the expansion opens up greater access to necessary procedures for patients that were once deemed high-risk for MR exams due to their implants, and that the change has been received well within the cardiology community. Such a switch, though, will take a while for radiologists to come around to, he warns.
Though expensive, MR can be cost effective when ruling
out or diagnosing diseases earlier and more accurately.
out or diagnosing diseases earlier and more accurately.
The bottom line
Increasing coverage of implants, along with AI, the cloud and gadolinium reduction are all expected to act as drivers in the increasing utilization of MR, along with increases in labor, consumable costs and services. The advent of new technologies such as higher strength magnets, nanotechnologies, and wide-bore and lighter-weighing systems will also make their mark, helping to drive the MR market from its current value of $5.8 billion at a rate of 3.9 percent annually.
“MR will always be a costly test,” said James Laskaris, a clinical strategist for MD Buyline. “But the key is it will be a cost avoidance technology by either ruling out or diagnosing a disease earlier and more accurately.”
Echoing this sentiment is Dr. Sriram Mannava, quality improvement and safety officer at Columbus Radiology Corporation, a Radiology Partners practice, who perceives utilization continuing to increase outside of outpatient settings and in ways in which MR is applied to those who require it.
“There is a really big push by payors right now for high-quality, low-cost imaging, and we’re seeing a lot less of hospital outpatient imaging because it costs so much more,” he said. “MR is not an inexpensive modality, but the information we get from it can be very useful. What we need to make sure of is that we are using it in an appropriate fashion.”