Optimizing PET and SPECT technology
June 15, 2020
by Lisa Chamoff, Contributing Reporter
Like the rest of the medical imaging industry, the PET and SPECT market has continued its focus on faster scans and decreased radiation dose. Manufacturers are also bringing more portable and mobile scanners to the market to increase access to scanners that are used to detect and treat cancer and cardiac issues, and diagnose neurodegenerative diseases, such as Parkinson's and Alzheimer's.
Here’s a look at what's new, and updates to what's emerging, in the space.
Canon Medical Systems USA
At last year’s RSNA annual meeting, Canon Medical Systems USA introduced its Cartesion Prime PET/CT system, a new premium Digital PET/CT scanner.
The scanner features a new detector built by Canon Medical that the company says provides 100% coverage, with one-to-one coupling and a large, 27-centimeter axial field of view, and a low time of flight, at 280 picoseconds, which may allow for faster scans at a lower dose.
“All of our crystal is covered by the detector (and) every individual small crystal can have its own SiPM (silicon photomultiplier) detector,” said Angela Dunaway, senior manager for molecular imaging solutions marketing for Canon Medical Systems USA. “We think from a technology perspective, we’ve really leveraged the value of SiPM.”
Perhaps the biggest new development is that the scanner forgoes the traditional water cooling system with a chiller for an air-cooled approach, which provides more dependability and allows the scanner to fit into smaller spaces.
The PET is combined with the company’s Aquilion Prime SP CT, which provides customers with access to new technologies on the CT side, Dunaway said. The stand-alone Aquilion Prime system recently received FDA clearance for Canon Medical’s AiCE Deep Learning Reconstruction.
“Most customers looking to move to digital PET/CT want to be set up for the future,” Dunaway said. “Canon Medical consistently migrates advanced technologies across product lines, providing our customers with more opportunities in improving patient care now and in the future.”
Cubresa
The company’s main clinical product is called the BrainPET, a PET insert that fits inside an MR scanner. The product was developed through a joint venture in China.
The first prototype of the product, the combination of a head coil with a PET ring wrapped around it, is going to be tested in a hospital in China, with the second prototype coming to Canada in the fall of this year.
There is interest in using the product for research in a variety of application areas including neurodegenerative diseases, brain cancer, epilepsy and infectious disease research, said Dr. James Schellenberg, founder and chief executive officer of Cubresa.
“However, we believe the key market for the BrainPET will be in the detection and treatment of Alzheimer’s disease ,” Schellenberg said.
NuPET, the company’s preclinical PET insert, has received interest from level 3 biosafety labs looking to research infectious diseases, such as COVID-19, Schellenberg said.
“I think there will be increased interest in imaging infectious diseases,” Schellenberg said. “NuPET can play an important role as it is useful for understanding the pathogenesis of viruses, and how vaccines and antivirals respond in animal models.”
Digirad
In the fourth quarter of 2019, Digirad released a new model of its portable Ergo Imaging System for general nuclear medicine.
The Ergo system used to have a laptop on a separate cart, while everything is integrated on the new product.
“The feedback we received from customers was that it would be easier to use if it were all-in-one,” said Martin Shirley, president of diagnostic services at Digirad. “So we took the components of a desktop computer and put it into the chassis of the Ergo system.”
The system also comes with a stationary camera for doing planar studies and replaces a lead acid battery with a lithium battery that provides two hours of standby time and one hour of performance, versus about 30 minutes of standby on the old system.
“With most bedside scans there are 30 minutes of actual imaging,” Shirley said. “You can still plug in if you want to, but now you don't have to.”
The company also upgraded its Cardius XPO Series of scanners – Cardius 2 XPO, the mobile Cardius 2M XPO and the Cardius 3 XPO, which comes with a third detector to reduce dose and imaging time – removing an umbilical connection to a cart to create one device with an integrated computer.
GE Healthcare
Last year, GE Healthcare debuted its Discovery IQ Gen 2 at the SNMMI 2019 Annual Meeting in Anaheim, California.
The second generation of the five-ring scanner has added MotionFree technology, which prevents blurring in images due to respiratory motion.
The company is also seeing its Discovery IQ Gen 2 and NM/CT 870 CZT used for theranostics, says Nathan Hermony, vice president and general manager for molecular imaging at GE Healthcare.
GE Healthcare, which also offers a series of cyclotrons, has an agreement to distribute a tracer called 18F Flurpiridaz that, once it’s cleared by the FDA, will enable scans that can measure both perfusion of heart and blood flow.
“When 18F Flurpiridaz is approved, I think there will be an increase in perfusion scans using PET,” Hermony said.
MiE
While awaiting FDA 510(k) clearance for a new stand-alone PET scanner later this year, MiE is offering an option to upgrade the acquisition and processing stations of existing PET ECAT SCINTRON series systems, used for cardiac PET imaging.
The upgrade doesn’t change the gantry’s hardware, but can enhance the software to allow the system to stay up to date with the current market, said Thomas Kühl, president of MiE — Gamma Camera and PET systems.
“The majority of MiE customers opted to purchase the SCINTRON due to the ability to perform robust 3D MPI (myocardial perfusion imaging) with a PET ECAT scanner,” Kühl said. “There have been multiple requests from cardiologists, using the ECAT series with SCINTRON, to have the option to perform simultaneous PET 3D myocardial blood flow (MBF) and 3D MPI using Rb-82.”
The dosing of Rb-82 can result in deadtime issues during the early MBF phase of the ECAT PET scanner family, Kühl said. A current solution is a 2D/3D acquisition protocol that retracts the 2D septa after the MBF phase and acquires the MPI in 3D mode. That motion can take up to 60 seconds between the two acquisitions. This is not ideal due to Rb-82’s short half-life, and can result in diminished image quality of perfusion imaging in some patients unless dose is increased.
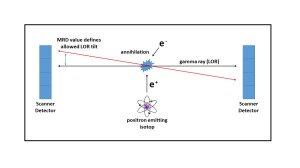
The company’s development team has come up with a solution called “MRD switching” that achieves a fully 3D acquisition workflow by using the SCINTRON acquisition hardware and software to avoid meeting or exceeding the limitations of the ECAT PET scanner family, Kühl said. The solution adapts the maximum ring distance (MRD) parameter of the scanner during the acquisition.
“It is still important to consider patient dose, but by having the software option to switch the MRD, other protocol concerns are avoided,” Kühl said.
MiE offers iterative reconstruction for 2D and 3D PET myocardial examinations. For customers wanting to perform simultaneous 3D flow and perfusion, the latest reconstruction speed-up upgrade is recommended to have a more efficient workflow, Kühl said. It also is helpful for customers using the ECAT HR+ system for perfusion imaging, since there are more lines of response that need to be considered during reconstruction.
MILabs
The company continues to develop its VECTor6 Broadband Photon Tomography preclinical imaging platform, working with clinical partners to test new applications of the technology, which has the capability to do simultaneous and quantitative imaging of co-injected PET tracers and multiple PET/SPECT tracers.
At Oxford University, for example, clinicians have used the platform to develop a method to very accurately view the uptake in tumor cells, versus the surrounding tissue.
“By imaging two tracers simultaneous one can measure the specific uptake in tumor cells,” said Frederik Beekman, chief executive officer of MILabs. “We see more interesting applications of PET/SPECT imaging for treating tumors.”
There is also the potential to use the system to develop a new generation of radiopharmaceuticals for the growing field of theranostics.
“A lot of our clients did first publications to show that this really works,” Beekman said.
RefleXion Medical
RefleXion Medical continues to develop its biology-guided radiotherapy (BgRT) system, which uses emissions from PET signals to guide therapy in real time.
The company is aiming to file for FDA clearance on the BgRT next year and is working with clinical partners to explore the use of PET and fan-beam CT, combined with a linear accelerator, as a treatment device for Stage 4 patients and those with metastatic disease.
“We’re using PET in a different way that turns tumors into real-time transponders,” said Sam Mazin, RefleXion Medical’s founder and chief technology officer. “With BgRT, you can enable all tumors to light up, which allows treating multiple tumors in the same session. We view it as a significant improvement to conventional radiotherapy today.”
Randomized controlled trials for localized treatment of limited metastatic disease in some cancers have shown improvement in overall survival of 30 to 50%, Mazin said.
Mazin said there has been interest among the pharmaceutical industry in combination therapy.
Philips
Philips is continuing to promote Vereos, the digital PET/CT system it began shipping at the end of 2017.
Shekar Ramakrishna, general manager for advanced molecular imaging at Philips, said that the company has now has more than four years of investigational studies and over 100 published clinical studies supporting the world’s first and only fully digital PET/CT solution.
"We're working to support hospitals and imaging centers as they seek to step up their performance by simultaneously improving the patient experience, health outcomes, and staff experience, while lowering the cost of care," Ramakrishna said.
Siemens Healthineers
Earlier this year, Siemens received FDA clearance for the new AIDAN artificial intelligence technologies for its Biograph family of PET/CT systems, including the Biograph Horizon, Biograph mCT, and Biograph Vision.
AIDAN provides three new features — FlowMotion AI, OncoFreeze AI and PET FAST WorkFlow AI.
FlowMotion AI enhances the company’s FlowMotion technology, which provides personalized exam protocols based on the patient’s anatomy,
“You design a protocol to a disease,” said Katherina Swystun, global PET marketing manager at Siemens Healthineers. “What FlowMotion AI allows you to do is set scan parameters based on disease, and it enables you to personalize the scan to the patient by automatically detecting the patient’s anatomy and defining the correct scan parameters and ranges specific to that patient. It gives you the flexibility to integrate, for example, motion management easily into your scan protocol. The AI will select automatically the range where motion management is needed and corrects for it.”
Oncofreeze AI creates an image that is virtually free of motion without the need for a respiratory belt.
“Positioning respiratory belts can be tricky,” Swystun said. “A patient’s breathing can change during acquisition. OncoFreeze AI is a deviceless solution without the need to prepare the patient and position a respiratory belt or alternate device. The AI portion will select automatically the range where motion generally occurs based on the patient’s anatomy resulting in an image virtually free of motion .”
Benefits come with using Oncofreeze AI and FlowMotion AI together.
“With both FlowMotion and OncoFreeze, the user would normally set up the ranges manually,” Swystun said. “Now with the introduction of AI we’ve taken that and made it automatic, so the user doesn’t have to choose where to start and stop the scan.”
FAST PET Workflow AI assists technologists after the exam by creating parallel ranges of reconstructed data to send to the PACS automatically, instead of requiring the technologist to do it manually.
“It will detect where the reconstructed images start and end, prepare the parallel ranges, such as fused axial, coronal and sagittal, and send them off automatically, rather than the user defining the ranges and sending them across manually,” Swystun said. “It streamlines it for them.”
On the SPECT side, the company recently received FDA 510(k) clearance for the inclusion of an additional isotope — Iodine-131, for use in evaluating and planning treatment – with xSPECT Quant.
As with all xSPECT Quant isotopes, a patented National Institute of Standards and Technology (NIST)-traceable calibration source is utilized and allows for the use of an expanded quantification solution available with the Symbia Intevo and Symbia Intevo Bold SPECT/CT scanners. Without xSPECT Quant, customers have to use phantoms and do calculations manually for calibration, which are time consuming and error-prone, said Collin Schaeffer, global marketing operations manager for PET/CT, SPECT, and SPECT/CT at Siemens Healthineers.
“It allows us to standardize the data across scanners,” Schaeffer said. “Physicians can evaluate how much dose has gone to certain areas of the body and this can help them plan for additional therapy, and aid in decision making for providing the right dose at the right time for each patient.”
Spectrum Dynamics
At the end of 2018, Spectrum Dynamics received FDA clearance for its VERITON-CT SPECT/CT system, with 16-, 64- or 128-slice configurations, following clearance of the VERITON-NM 12-detector digital SPECT system.
Both the VERITON-NM and the VERITON-CT 64 have a unique multi-detector 360-degree geometry that can get closer to the patient and organ of interest and yields three times the volumetric sensitivity and twice the throughput of conventional dual-head analog SPECT cameras, according to the company.
"In nuclear medicine, proximity, 3D coverage and shorter scan times are key to exam quality and patient satisfaction," said Nilda Rivera, senior director of Global Marketing for Spectrum Dynamics.
The company has installed several of the CT-enabled systems, which provides all the capabilities of a CT scanner, for general and cardiac applications and nuclear medicine scanning for diagnosis of diseases, such as Parkinson's, myocardial perfusion, bone infections.
United Imaging
Last year, the Shanghai-based imaging company introduced the first mobile digital PET/CT scanner in the U.S.
United Imaging teamed up with Shared Medical Services (SMS) to deploy its uMI 550 digital PET/CT systems on one of SMS’s mobile diagnostic imaging vehicles. The product is geared toward hospitals that can’t justify the purchase of a full-time PET-CT system, or health systems that need the flexibility of PET in multiple locations for their patients.
The scanner has the same technology as its uEXPLORER total-body PET/CT scanner, which received FDA clearance at the beginning of 2019 and is the first scanner that can do a 3D full-body scan in one image capture.
“We’re focused on equal healthcare for all and bringing the best technology we can to every price point,” said Jeffrey Bundy, chief executive officer of UIH Solutions LLC. “The mobile digital PET/CT (uMI 550) is emblematic of who we are as a company because it’s taking the same kind of digital platform previously available only in large institutions and bringing that to small communities on a mobile route.”
In May of last year, United Imaging received FDA clearance for its uPMR 790 HD TOF PET/MR system, which also uses the same digital platform as its other molecular imaging scanners. It’s designed for certain conditions that benefit from the simultaneous acquisition of MR during a PET scan.
The company also continues to work with its clinical partners to share research on use of its uEXPLORER. Studies underway at facilities such as UC Davis and overseas have looked at performing higher-resolution scans while keeping scan times the same. The company is currently manufacturing the first systems at its Houston facility and has also engaged in a strategic partnership with BAMF Health, the first organization in the United States to purchase United Imaging's uEXPLORER and uPMR 790 PET/MR for clinical use.
The company also received FDA clearance this past January for its HYPER-Iterative reconstruction technology, which Bundy said brings noise control into each iteration and allows for consistent improvement of lesion detectability and quantitative accuracy, regardless of patient type. This feature is now standard on the uMI 550 and is rolling out to the rest of the PET portfolio in the coming months.
Another technology that is aimed at future imaging and workflow improvements and that uses a deep learning approach, called HYPER DLR, is under consideration by the FDA.
Here’s a look at what's new, and updates to what's emerging, in the space.
Canon Medical Systems USA
At last year’s RSNA annual meeting, Canon Medical Systems USA introduced its Cartesion Prime PET/CT system, a new premium Digital PET/CT scanner.
The scanner features a new detector built by Canon Medical that the company says provides 100% coverage, with one-to-one coupling and a large, 27-centimeter axial field of view, and a low time of flight, at 280 picoseconds, which may allow for faster scans at a lower dose.
“All of our crystal is covered by the detector (and) every individual small crystal can have its own SiPM (silicon photomultiplier) detector,” said Angela Dunaway, senior manager for molecular imaging solutions marketing for Canon Medical Systems USA. “We think from a technology perspective, we’ve really leveraged the value of SiPM.”
Perhaps the biggest new development is that the scanner forgoes the traditional water cooling system with a chiller for an air-cooled approach, which provides more dependability and allows the scanner to fit into smaller spaces.
The PET is combined with the company’s Aquilion Prime SP CT, which provides customers with access to new technologies on the CT side, Dunaway said. The stand-alone Aquilion Prime system recently received FDA clearance for Canon Medical’s AiCE Deep Learning Reconstruction.
“Most customers looking to move to digital PET/CT want to be set up for the future,” Dunaway said. “Canon Medical consistently migrates advanced technologies across product lines, providing our customers with more opportunities in improving patient care now and in the future.”
Cubresa
The company’s main clinical product is called the BrainPET, a PET insert that fits inside an MR scanner. The product was developed through a joint venture in China.
The first prototype of the product, the combination of a head coil with a PET ring wrapped around it, is going to be tested in a hospital in China, with the second prototype coming to Canada in the fall of this year.
There is interest in using the product for research in a variety of application areas including neurodegenerative diseases, brain cancer, epilepsy and infectious disease research, said Dr. James Schellenberg, founder and chief executive officer of Cubresa.
“However, we believe the key market for the BrainPET will be in the detection and treatment of Alzheimer’s disease ,” Schellenberg said.
NuPET, the company’s preclinical PET insert, has received interest from level 3 biosafety labs looking to research infectious diseases, such as COVID-19, Schellenberg said.
“I think there will be increased interest in imaging infectious diseases,” Schellenberg said. “NuPET can play an important role as it is useful for understanding the pathogenesis of viruses, and how vaccines and antivirals respond in animal models.”
Digirad
In the fourth quarter of 2019, Digirad released a new model of its portable Ergo Imaging System for general nuclear medicine.
The Ergo system used to have a laptop on a separate cart, while everything is integrated on the new product.
“The feedback we received from customers was that it would be easier to use if it were all-in-one,” said Martin Shirley, president of diagnostic services at Digirad. “So we took the components of a desktop computer and put it into the chassis of the Ergo system.”
The system also comes with a stationary camera for doing planar studies and replaces a lead acid battery with a lithium battery that provides two hours of standby time and one hour of performance, versus about 30 minutes of standby on the old system.
“With most bedside scans there are 30 minutes of actual imaging,” Shirley said. “You can still plug in if you want to, but now you don't have to.”
The company also upgraded its Cardius XPO Series of scanners – Cardius 2 XPO, the mobile Cardius 2M XPO and the Cardius 3 XPO, which comes with a third detector to reduce dose and imaging time – removing an umbilical connection to a cart to create one device with an integrated computer.
GE Healthcare
Last year, GE Healthcare debuted its Discovery IQ Gen 2 at the SNMMI 2019 Annual Meeting in Anaheim, California.
The second generation of the five-ring scanner has added MotionFree technology, which prevents blurring in images due to respiratory motion.
The company is also seeing its Discovery IQ Gen 2 and NM/CT 870 CZT used for theranostics, says Nathan Hermony, vice president and general manager for molecular imaging at GE Healthcare.
GE Healthcare, which also offers a series of cyclotrons, has an agreement to distribute a tracer called 18F Flurpiridaz that, once it’s cleared by the FDA, will enable scans that can measure both perfusion of heart and blood flow.
“When 18F Flurpiridaz is approved, I think there will be an increase in perfusion scans using PET,” Hermony said.
MiE
While awaiting FDA 510(k) clearance for a new stand-alone PET scanner later this year, MiE is offering an option to upgrade the acquisition and processing stations of existing PET ECAT SCINTRON series systems, used for cardiac PET imaging.
The upgrade doesn’t change the gantry’s hardware, but can enhance the software to allow the system to stay up to date with the current market, said Thomas Kühl, president of MiE — Gamma Camera and PET systems.
“The majority of MiE customers opted to purchase the SCINTRON due to the ability to perform robust 3D MPI (myocardial perfusion imaging) with a PET ECAT scanner,” Kühl said. “There have been multiple requests from cardiologists, using the ECAT series with SCINTRON, to have the option to perform simultaneous PET 3D myocardial blood flow (MBF) and 3D MPI using Rb-82.”
The dosing of Rb-82 can result in deadtime issues during the early MBF phase of the ECAT PET scanner family, Kühl said. A current solution is a 2D/3D acquisition protocol that retracts the 2D septa after the MBF phase and acquires the MPI in 3D mode. That motion can take up to 60 seconds between the two acquisitions. This is not ideal due to Rb-82’s short half-life, and can result in diminished image quality of perfusion imaging in some patients unless dose is increased.
The company’s development team has come up with a solution called “MRD switching” that achieves a fully 3D acquisition workflow by using the SCINTRON acquisition hardware and software to avoid meeting or exceeding the limitations of the ECAT PET scanner family, Kühl said. The solution adapts the maximum ring distance (MRD) parameter of the scanner during the acquisition.
“It is still important to consider patient dose, but by having the software option to switch the MRD, other protocol concerns are avoided,” Kühl said.
MiE offers iterative reconstruction for 2D and 3D PET myocardial examinations. For customers wanting to perform simultaneous 3D flow and perfusion, the latest reconstruction speed-up upgrade is recommended to have a more efficient workflow, Kühl said. It also is helpful for customers using the ECAT HR+ system for perfusion imaging, since there are more lines of response that need to be considered during reconstruction.
MILabs
The company continues to develop its VECTor6 Broadband Photon Tomography preclinical imaging platform, working with clinical partners to test new applications of the technology, which has the capability to do simultaneous and quantitative imaging of co-injected PET tracers and multiple PET/SPECT tracers.
At Oxford University, for example, clinicians have used the platform to develop a method to very accurately view the uptake in tumor cells, versus the surrounding tissue.
“By imaging two tracers simultaneous one can measure the specific uptake in tumor cells,” said Frederik Beekman, chief executive officer of MILabs. “We see more interesting applications of PET/SPECT imaging for treating tumors.”
There is also the potential to use the system to develop a new generation of radiopharmaceuticals for the growing field of theranostics.
“A lot of our clients did first publications to show that this really works,” Beekman said.
RefleXion Medical
RefleXion Medical continues to develop its biology-guided radiotherapy (BgRT) system, which uses emissions from PET signals to guide therapy in real time.
The company is aiming to file for FDA clearance on the BgRT next year and is working with clinical partners to explore the use of PET and fan-beam CT, combined with a linear accelerator, as a treatment device for Stage 4 patients and those with metastatic disease.
“We’re using PET in a different way that turns tumors into real-time transponders,” said Sam Mazin, RefleXion Medical’s founder and chief technology officer. “With BgRT, you can enable all tumors to light up, which allows treating multiple tumors in the same session. We view it as a significant improvement to conventional radiotherapy today.”
Randomized controlled trials for localized treatment of limited metastatic disease in some cancers have shown improvement in overall survival of 30 to 50%, Mazin said.
Mazin said there has been interest among the pharmaceutical industry in combination therapy.
Philips
Philips is continuing to promote Vereos, the digital PET/CT system it began shipping at the end of 2017.
Shekar Ramakrishna, general manager for advanced molecular imaging at Philips, said that the company has now has more than four years of investigational studies and over 100 published clinical studies supporting the world’s first and only fully digital PET/CT solution.
"We're working to support hospitals and imaging centers as they seek to step up their performance by simultaneously improving the patient experience, health outcomes, and staff experience, while lowering the cost of care," Ramakrishna said.
Siemens Healthineers
Earlier this year, Siemens received FDA clearance for the new AIDAN artificial intelligence technologies for its Biograph family of PET/CT systems, including the Biograph Horizon, Biograph mCT, and Biograph Vision.
AIDAN provides three new features — FlowMotion AI, OncoFreeze AI and PET FAST WorkFlow AI.
FlowMotion AI enhances the company’s FlowMotion technology, which provides personalized exam protocols based on the patient’s anatomy,
“You design a protocol to a disease,” said Katherina Swystun, global PET marketing manager at Siemens Healthineers. “What FlowMotion AI allows you to do is set scan parameters based on disease, and it enables you to personalize the scan to the patient by automatically detecting the patient’s anatomy and defining the correct scan parameters and ranges specific to that patient. It gives you the flexibility to integrate, for example, motion management easily into your scan protocol. The AI will select automatically the range where motion management is needed and corrects for it.”
Oncofreeze AI creates an image that is virtually free of motion without the need for a respiratory belt.
“Positioning respiratory belts can be tricky,” Swystun said. “A patient’s breathing can change during acquisition. OncoFreeze AI is a deviceless solution without the need to prepare the patient and position a respiratory belt or alternate device. The AI portion will select automatically the range where motion generally occurs based on the patient’s anatomy resulting in an image virtually free of motion .”
Benefits come with using Oncofreeze AI and FlowMotion AI together.
“With both FlowMotion and OncoFreeze, the user would normally set up the ranges manually,” Swystun said. “Now with the introduction of AI we’ve taken that and made it automatic, so the user doesn’t have to choose where to start and stop the scan.”
FAST PET Workflow AI assists technologists after the exam by creating parallel ranges of reconstructed data to send to the PACS automatically, instead of requiring the technologist to do it manually.
“It will detect where the reconstructed images start and end, prepare the parallel ranges, such as fused axial, coronal and sagittal, and send them off automatically, rather than the user defining the ranges and sending them across manually,” Swystun said. “It streamlines it for them.”
On the SPECT side, the company recently received FDA 510(k) clearance for the inclusion of an additional isotope — Iodine-131, for use in evaluating and planning treatment – with xSPECT Quant.
As with all xSPECT Quant isotopes, a patented National Institute of Standards and Technology (NIST)-traceable calibration source is utilized and allows for the use of an expanded quantification solution available with the Symbia Intevo and Symbia Intevo Bold SPECT/CT scanners. Without xSPECT Quant, customers have to use phantoms and do calculations manually for calibration, which are time consuming and error-prone, said Collin Schaeffer, global marketing operations manager for PET/CT, SPECT, and SPECT/CT at Siemens Healthineers.
“It allows us to standardize the data across scanners,” Schaeffer said. “Physicians can evaluate how much dose has gone to certain areas of the body and this can help them plan for additional therapy, and aid in decision making for providing the right dose at the right time for each patient.”
Spectrum Dynamics
At the end of 2018, Spectrum Dynamics received FDA clearance for its VERITON-CT SPECT/CT system, with 16-, 64- or 128-slice configurations, following clearance of the VERITON-NM 12-detector digital SPECT system.
Both the VERITON-NM and the VERITON-CT 64 have a unique multi-detector 360-degree geometry that can get closer to the patient and organ of interest and yields three times the volumetric sensitivity and twice the throughput of conventional dual-head analog SPECT cameras, according to the company.
"In nuclear medicine, proximity, 3D coverage and shorter scan times are key to exam quality and patient satisfaction," said Nilda Rivera, senior director of Global Marketing for Spectrum Dynamics.
The company has installed several of the CT-enabled systems, which provides all the capabilities of a CT scanner, for general and cardiac applications and nuclear medicine scanning for diagnosis of diseases, such as Parkinson's, myocardial perfusion, bone infections.
United Imaging
Last year, the Shanghai-based imaging company introduced the first mobile digital PET/CT scanner in the U.S.
United Imaging teamed up with Shared Medical Services (SMS) to deploy its uMI 550 digital PET/CT systems on one of SMS’s mobile diagnostic imaging vehicles. The product is geared toward hospitals that can’t justify the purchase of a full-time PET-CT system, or health systems that need the flexibility of PET in multiple locations for their patients.
The scanner has the same technology as its uEXPLORER total-body PET/CT scanner, which received FDA clearance at the beginning of 2019 and is the first scanner that can do a 3D full-body scan in one image capture.
“We’re focused on equal healthcare for all and bringing the best technology we can to every price point,” said Jeffrey Bundy, chief executive officer of UIH Solutions LLC. “The mobile digital PET/CT (uMI 550) is emblematic of who we are as a company because it’s taking the same kind of digital platform previously available only in large institutions and bringing that to small communities on a mobile route.”
In May of last year, United Imaging received FDA clearance for its uPMR 790 HD TOF PET/MR system, which also uses the same digital platform as its other molecular imaging scanners. It’s designed for certain conditions that benefit from the simultaneous acquisition of MR during a PET scan.
The company also continues to work with its clinical partners to share research on use of its uEXPLORER. Studies underway at facilities such as UC Davis and overseas have looked at performing higher-resolution scans while keeping scan times the same. The company is currently manufacturing the first systems at its Houston facility and has also engaged in a strategic partnership with BAMF Health, the first organization in the United States to purchase United Imaging's uEXPLORER and uPMR 790 PET/MR for clinical use.
The company also received FDA clearance this past January for its HYPER-Iterative reconstruction technology, which Bundy said brings noise control into each iteration and allows for consistent improvement of lesion detectability and quantitative accuracy, regardless of patient type. This feature is now standard on the uMI 550 and is rolling out to the rest of the PET portfolio in the coming months.
Another technology that is aimed at future imaging and workflow improvements and that uses a deep learning approach, called HYPER DLR, is under consideration by the FDA.