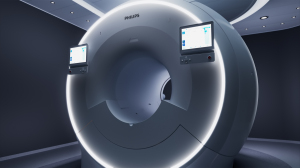
FDA greenlights Philips' 7700 3T MR system
May 09, 2022
by John R. Fischer, Senior Reporter
Philips has scored FDA clearance for its 7700 3T MR system, designed with powerful XP gradients for improved high-quality diffusion imaging.
The solution is equipped with six multi-nuclei capabilities that increase diagnostic confidence for conducting and interpreting neurological scans and add important metabolic information to exams. The system made its debut at the International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting, taking place May 7 to May 12 in London. It will also be spotlighted at the European Congress of Radiology (ECR) annual congress in Vienna in July.
The system has a 65-20 gradient chain. Its XP gradient coils are able to achieve up to 35% higher signal-to-noise ratios and reduce scanning time by up to 35%. This enables radiologists to complete 20% more fMR volumes and 50% more diffusion tensor imaging directions to produce detailed high-resolution images that they can use to confidently identify and characterize lesions.
Another valuable component is in-built AI applications such as touchless patient sensing and motion detection for addressing upswings in patient volumes that many radiology departments currently experience. “The low effort required for modifying scan parameters and protocols supports fast and easy experimentation with imaging techniques. These latest features clearly help improve our patient and staff experience and distinguish Philips as one of the main reasons we choose this system,” said Walter Heindel, professor of radiology and chairman of the department of radiology at the University Hospital Münster in Germany, in a statement.
The MR 7700 can perform conventional proton MR and spectroscopy and has in-built protocols to combine proton scanning with other forms of imaging based on sodium, phosphorus, carbon, fluorine and xenon. This multi-nuclei capability makes the system a good option for anatomical and metabolic/functional imaging in neurology, pulmonology and oncology. “For every nucleus, you would get a little bit of different information. You get incremental information, and being able to get a full data set across the six, multi-nuclei gives you an opportunity to start with proton and potentially expand into the other five and get a complete picture with more information. Doctors love more information because that will help them get to a better diagnosis,” Arjen Radder, general manager of magnetic resonance and diagnostic X-ray at Philips, told HCB News.
Applications include 23Na sodium imaging for measuring metabolic rates in the brain (neurodegenerative diseases, stroke and ischemia); assessing cartilage with 31P-MRS in phosphorus MR spectroscopy to measure the energy metabolism in the brain (onco, hemodynamic impairment), the muscle, the liver and the heart. Hyperpolarized 13C spectroscopy can also be used to measure metabolism in oncology and diabetes patients.
Through the integration of a dual-tuned head coil, both multi-nuclei and conventional protocol MR exams can be performed on the same coil and within the same user interface. This increases workflow efficiency. AI-assisted workflow solutions also keep exams on schedule, which improves both patient and staff experience. The use of its AI-based smart connected imaging capabilities, optimized workflows and integrated clinical solutions helps it meet the quadruple aim by improving MR department productivity, enhancing the patient and staff experience and delivering high-quality diagnostic outcomes.
The XP gradients are expected to be helpful in research programs on neuroscience progress without compromising workflow efficiency or wide-bore patient comfort. Additionally, users can more easily switch from clinical to research protocols just by selecting a parameter in their regular protocols on the interface. The solution provides the necessary codes for each protocol. “At any point of the day, they can decide if they want to run a patient on the clinical protocol or they will run it on a research protocol and they don't have to reconfigure. They don't have to re-optimize the system. They don't have to select specific protocols in a cumbersome way. It's all easy. It's all ready to go,” said Radder.
The solution is part of the Philips Radiology portfolio of scalable high-performance MR systems, including its MR 5300 1.5T helium-free in operations MR scanner. All of these systems have intelligent software for automating tasks and technologist- and radiologist-centered image processing applications to increase diagnostic confidence. They also have enterprise-integrated workflows that streamline the pathway for precision care.
The MR 7700 is also CE marked and can be monitored 24/7.
The solution is equipped with six multi-nuclei capabilities that increase diagnostic confidence for conducting and interpreting neurological scans and add important metabolic information to exams. The system made its debut at the International Society for Magnetic Resonance in Medicine (ISMRM) annual meeting, taking place May 7 to May 12 in London. It will also be spotlighted at the European Congress of Radiology (ECR) annual congress in Vienna in July.
The system has a 65-20 gradient chain. Its XP gradient coils are able to achieve up to 35% higher signal-to-noise ratios and reduce scanning time by up to 35%. This enables radiologists to complete 20% more fMR volumes and 50% more diffusion tensor imaging directions to produce detailed high-resolution images that they can use to confidently identify and characterize lesions.
Another valuable component is in-built AI applications such as touchless patient sensing and motion detection for addressing upswings in patient volumes that many radiology departments currently experience. “The low effort required for modifying scan parameters and protocols supports fast and easy experimentation with imaging techniques. These latest features clearly help improve our patient and staff experience and distinguish Philips as one of the main reasons we choose this system,” said Walter Heindel, professor of radiology and chairman of the department of radiology at the University Hospital Münster in Germany, in a statement.
The MR 7700 can perform conventional proton MR and spectroscopy and has in-built protocols to combine proton scanning with other forms of imaging based on sodium, phosphorus, carbon, fluorine and xenon. This multi-nuclei capability makes the system a good option for anatomical and metabolic/functional imaging in neurology, pulmonology and oncology. “For every nucleus, you would get a little bit of different information. You get incremental information, and being able to get a full data set across the six, multi-nuclei gives you an opportunity to start with proton and potentially expand into the other five and get a complete picture with more information. Doctors love more information because that will help them get to a better diagnosis,” Arjen Radder, general manager of magnetic resonance and diagnostic X-ray at Philips, told HCB News.
Applications include 23Na sodium imaging for measuring metabolic rates in the brain (neurodegenerative diseases, stroke and ischemia); assessing cartilage with 31P-MRS in phosphorus MR spectroscopy to measure the energy metabolism in the brain (onco, hemodynamic impairment), the muscle, the liver and the heart. Hyperpolarized 13C spectroscopy can also be used to measure metabolism in oncology and diabetes patients.
Through the integration of a dual-tuned head coil, both multi-nuclei and conventional protocol MR exams can be performed on the same coil and within the same user interface. This increases workflow efficiency. AI-assisted workflow solutions also keep exams on schedule, which improves both patient and staff experience. The use of its AI-based smart connected imaging capabilities, optimized workflows and integrated clinical solutions helps it meet the quadruple aim by improving MR department productivity, enhancing the patient and staff experience and delivering high-quality diagnostic outcomes.
The XP gradients are expected to be helpful in research programs on neuroscience progress without compromising workflow efficiency or wide-bore patient comfort. Additionally, users can more easily switch from clinical to research protocols just by selecting a parameter in their regular protocols on the interface. The solution provides the necessary codes for each protocol. “At any point of the day, they can decide if they want to run a patient on the clinical protocol or they will run it on a research protocol and they don't have to reconfigure. They don't have to re-optimize the system. They don't have to select specific protocols in a cumbersome way. It's all easy. It's all ready to go,” said Radder.
The solution is part of the Philips Radiology portfolio of scalable high-performance MR systems, including its MR 5300 1.5T helium-free in operations MR scanner. All of these systems have intelligent software for automating tasks and technologist- and radiologist-centered image processing applications to increase diagnostic confidence. They also have enterprise-integrated workflows that streamline the pathway for precision care.
The MR 7700 is also CE marked and can be monitored 24/7.