by
John R. Fischer, Senior Reporter | June 06, 2022
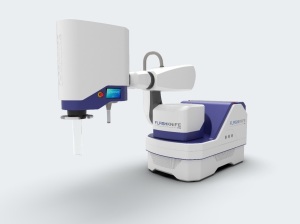
The FLASHKNiFE Consortium aims to make the FLASHKNiFE system clinically available.
PMB, a subsidiary of industrial company Alcen, has launched the FLASHKNiFE Consortium, a public-private partnership aiming to bring the FLASHKNiFE platform into clinical practice to provide patients with access to the ultra-fast treatment that is FLASH therapy.
Designed to deliver treatment in a matter of seconds in one single session, FLASH therapy spares patients from the side effects of conventional radiotherapy.
FLASHKNiFE has been tested in clinical applications such as skin cancer treatment and intraoperative radiotherapy. Supported by the European Institute of Innovation & Technology for Health (EIT Health), the FLASHKNiFE Consortium seeks to use the solution to reduce toxicity that leads to side effects, while increasing dosage that can be administered to tumors in order to treat patients who are resistant to conventional radiotherapy.
"The decision to embark on this very exciting EIT Health project was made because it brought together a biological innovation, the FLASH concept; a technology innovation, FLASHKNiFE; and the ability to turn these innovations into reality for the patient,” said professor Eric Deutsch, head of Gustave Roussy radiotherapy department, in a statement.
The consortium is made up of high-profile institutes across the continent, including PMB, Alcen, Institut Gustave Roussy, Centro Hospitalar Lisboa Norte, Universitätsklinikum Erlangen and ProductLife Group. Along with its clinical partner, Lausanne University Hospital (CHUV) in Switzerland, the consortium plans to conduct a multicentric clinical investigation with FLASHKNiFE for skin cancer starting in 2023.
Launched in 2020, FLASHKNiFE consists of an ultrahigh dose rate LINAC mounted on a mobile base, and combines usability, flexibility and FLASH delivery mode with precise configuration and monitoring capabilities. It is based on a prototype LINAC, Oriatron 6e, which accelerates electrons in FLASH mode.
FLASHKNiFE has an energy level of 10 MeV, which increases and improves penetration to treat the deepest tumors. It also orients and positions the beam source in relation to the patient.
The consortium has a total budget of €8.2 million and is co-funded by the EIT Health (€2.5 million) and the partners.
