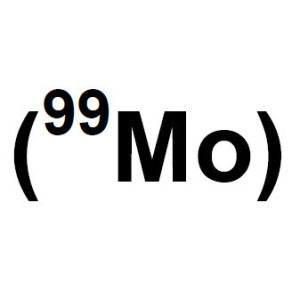by
Brendon Nafziger, DOTmed News Associate Editor | December 08, 2010
In a move called for by non-proliferation advocates, the United States received its first commercial shipment of molybdenum-99 made from low-enriched uranium on Monday.
The medical isotope is typically made from highly enriched uranium, which can be used to make weapons.
"Years ago we needed [highly enriched uranium] to provide the isotopes for diagnosis and treatment of disease. Now we can do it without using highly sensitive nuclear materials," Gary Samore, the coordinator for arms control at the White House, said in a statement.



Ad Statistics
Times Displayed: 174170
Times Visited: 3178 For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.
On Tuesday, Lantheus Medical Imaging, which received the shipment, said it started the first commercial production of the generators that convert the isotope into technetium-99, the daughter isotope used in imaging.
The new batch of moly shows that medical isotopes can be made from non-weapons-grade uranium and undermines Iran's arguments that it needs to HEU for medical purposes, Dr. Robert Atcher, past president of SNM and chairman of the group's Medical Isotopes Task Force, told Reuters.
The shipment came from NTP Radioisotopes Ltd., a subsidiary of Nesca, a South African company that created the isotope on its reactor using LEU. Nesca and its partner, Australian Nuclear Science and Technology Organisation, received a $25 million grant from the U.S. Government to make more low-LEU moly. The Nesca reactor is the only plant that currently operates with this technique, according to a November report from the Independent newspaper.
An earlier shipment of the LEU-made moly for testing was delivered to the United States in July. Lantheus received U.S. Food and Drug Administration approval for the drug in September, the N. Billerica, Mass.-based company told DOTmed News.
The company's TechneLite generators "milk" moly into its daughter isotope technetium-99, used in around 80 percent of the 50 million nuclear medicine scans performed worldwide each year, according to the Independent.
The LEU-made moly is thought to be more expensive to produce, according to the Independent. Lantheus said its policy was not to release confidential pricing information.