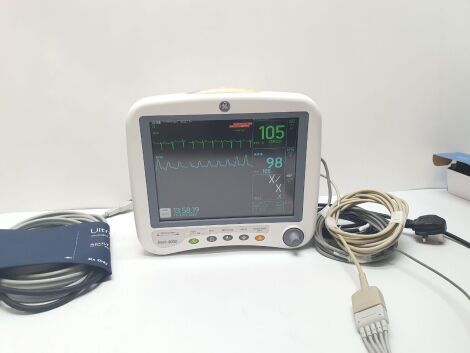
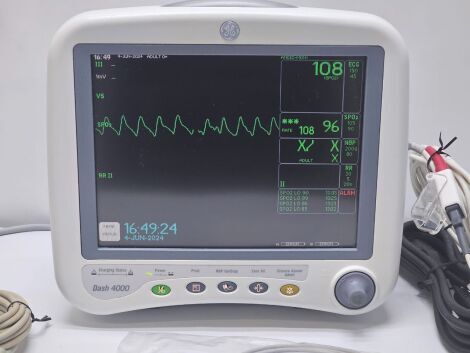
Patient Monitor GE Dash 4000 SpO2,ECG,NIBP Options Nellcor SpO2
The monitor is in good condition and working order
Tested and all parameters are within the limits
GE Gash 4000 Patient Monitor
Supplied with
1 x ECG lead
1 x Spo2 Sensor and adapter(adult)
1 x NIBP Hose and Cuff(adult)
1 x UK power cable
Few age related marks/please see all images
No batteries/Mains only monitor
Note: Import VAT,Duties,Taxes and any other import related charges are not included in the item price or shipping cost. These charges are the buyer's responsibility.
Note: Please check with your country's customs office to determine what these additional costs will be prior to bidding or buying.
Note: If the international shipping price is not listed in the listing, please contact us for a quote or with any questions.
Each device is professionally cleaned before dispatch
CE MARKED - This product is in compliance with the essential requirements of Council Directive
93/42/EEC Medical Device Directive, as amended by Council Directive 2007/47/EC, class IIa
UK -This device meets the requirements of the Medical Devices Regulations 2002.
DISCLAIMER: Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or consequential, associated in any way with the equipment. The purchaser, by purchasing this equipment, indicates their acknowledgment of, and agreement to the terms of this disclaimer.
Note: All medical items are sold to the best of our testing facilities, it will be the buyers responsibility to have these items tested/certified/calibrated before clinical use
US -The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item
Return Policy
Items are sold as-is with no returns or refunds available unless explicitly stated.