Please call if you have trouble purchasing on-line.
Exogen 4000+ with 100-200 prior uses, in excellent gently used condition. Free shipping.
Guaranteed to function for 175 additional treatments or 6 months, whichever comes first.
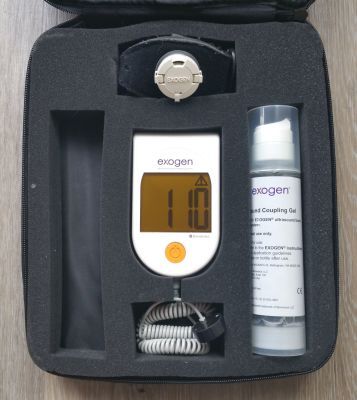
Includes main operating unit, strap, one bottle of gel, manual and case.
The Exogen Ultrasound Bone Stimulator uses ultrasound technology to speed up the healing of a broken or fractured bone by 38%. The device is very easy to use. The transducer is placed over your fracture and uses painless ultrasound technology over a 20-minute treatment to help stimulate bone growth and speed up healing.
We know you need this; so we'll process your order and ship very fast, often the same day!
USPS Priority Mail or equivalent UPS shipping is included for free. FedEx overnight shipping is available for $120, 2-day delivery is $60. Please call if needed or tell us in the comments when you purchase, and we'll contact you to make the arrangements.
Unit is sold with a 100% full replacement guarantee for 6 months or 175 additional treatments, whichever comes first.
Pictures shown are representative only.
There are no known contraindications for the Exogen device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel.
FDA requires that this device be used under doctor's care, and supervision. The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.
Return Policy
See listing description for return policy.