FREE SHIPPING with NEW Battery
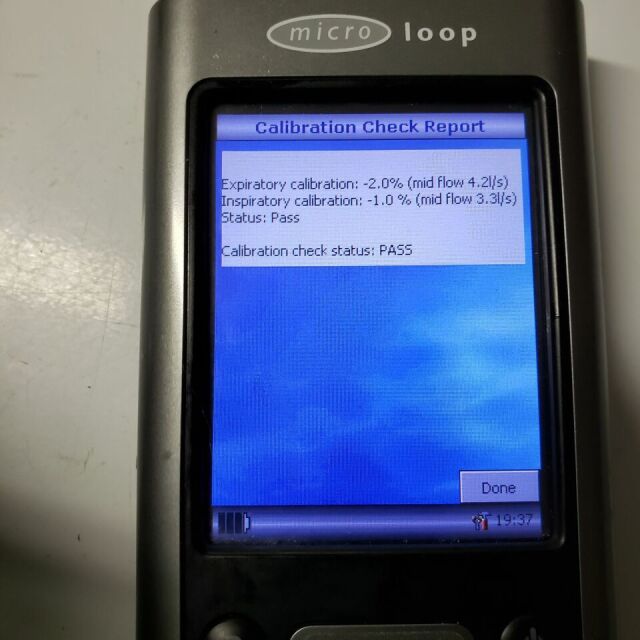
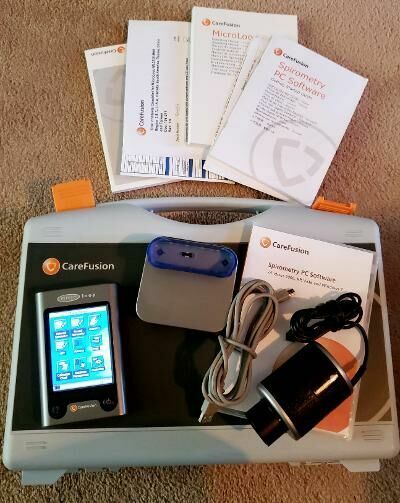
This spirometer was successfully calibrated by the seller immediately prior to this listing using a 3 L air syringe (for sale separately) designed for respiratory device calibration. The MicroLoop has no apparent issues. The battery retained its charge for four months while on our inventory shelf. At the buyers direction we will replace this battery with a new one or include a new battery in the package. Included is everything seen in the ad pictures plus a cradle/PC USB cable and a warranty that covers the device's passing a biomedical inspection after delivery. Micro Direct offers a PDF version of the MicroLoop operating manual at no charge at the MDSpiro.com website. This is a current product distributed worldwide by UK based Micro Direct. At the time of this unit's production Micro Direct was a subsidiary of CareFusion. Currrently Cardinal Health appears to own Micro Direct.
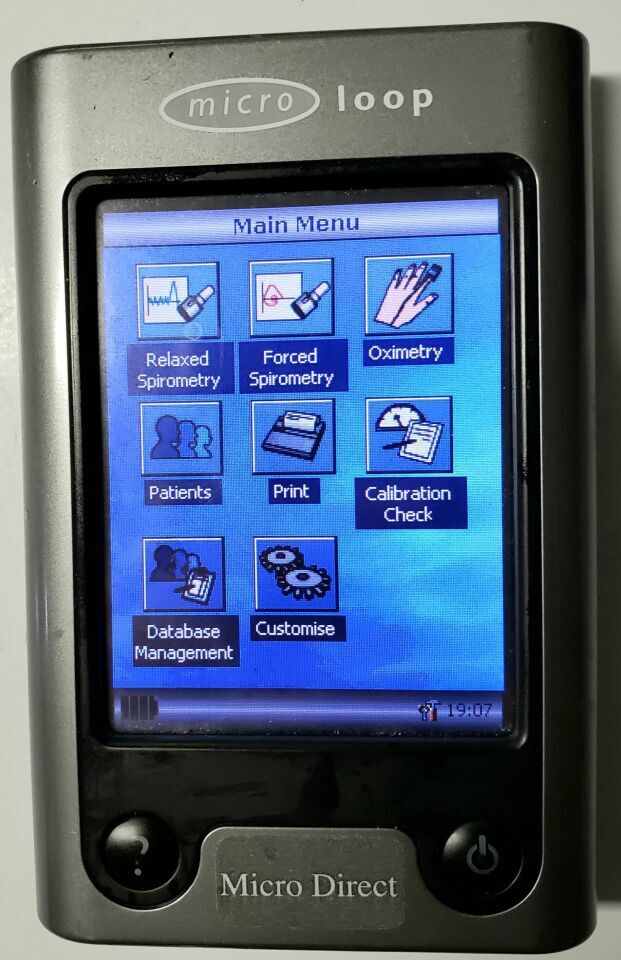
Touch screen color display
Fully customizable printout format
Direct connect to HP printers
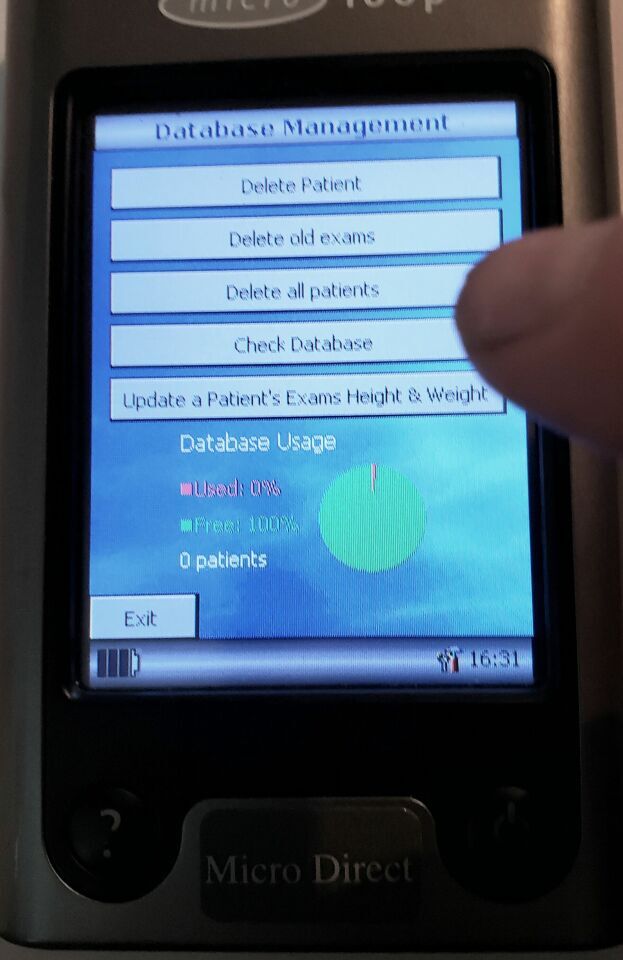
2000 Patient test memory
On Screen test quality prompts
SPIDA 5 Software included
Suited to measuring the very low flow rates of patients with COPD
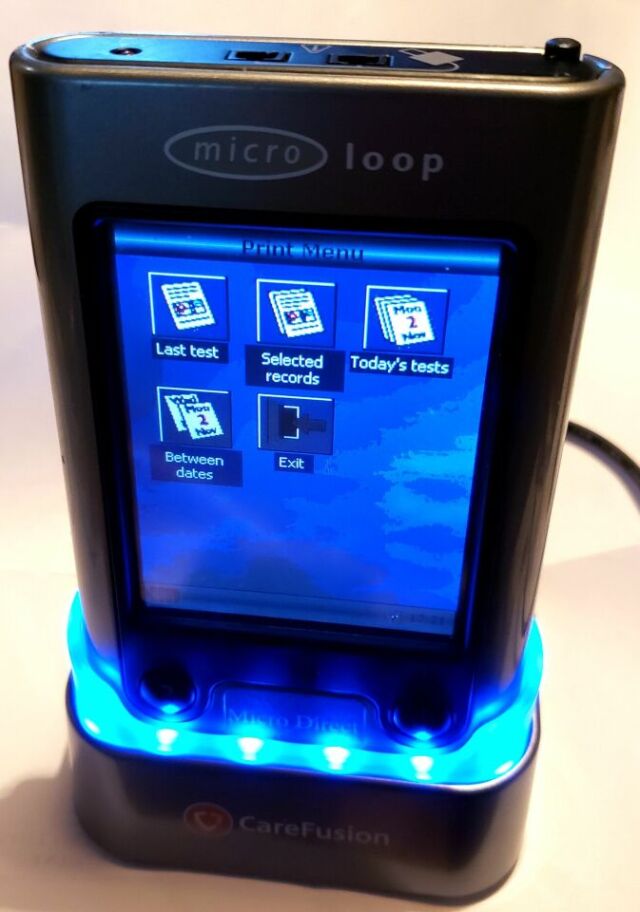
The MicroLoop is a battery operated portable spirometer with the unique combination of ease of use and sophistication. Ease of use is assured through the use of context sensitive help screens, accessed at a touch of a button, that explain every MicroLoop feature. The MicroLoop is supplied with a cradle that may be connected with the USB cables supplied, to either a PC or a printer. The cradle also connects to the mains adapter so that the MicroLoop’s batteries may be charged while it is placed in the cradle. The blue lights on the cradle indicate that it is being powered either by a PC connection or by the mains adapter. When either of these sources of power is connected to the cradle, it is ready to charge your MicroLoop. The MicroLoop utilizes a single patient use disposable mouthpiece or filter that must be disposed of after use.
The MicroLoop uses a Digital Volume Transducer, an extremely stable form of volume transducer, which measures expired air directly at B.T.P.S (Body Temperature and Pressure with Saturated water vapor) thus avoiding the inaccuracies of temperature corrections. The transducer is insensitive to the effects of condensation and temperature and avoids the need for individual calibration prior to performing a test. Test results may be uploaded to a PC using Spirometry PC Software and patient details may be downloaded to the MicroLoop.
The MicroLoop spirometer is intended for prescription use only, to measure the maximal volume and flow of air that can be moved in and out of a patient’s lungs. The system is intended for use with pediatric (4 to 17 years of age) and adult (18 to 99 years of age) patients in hospitals, physician offices, laboratories and occupational health testing environments.
System options allow you to configure the following:
Language
Height and weight units
Date format
Date separator
Color or monochrome printing (on external printer)
Personalized printout heading
Spirometry options allow you to configure the following:
Relaxed spirometry mode (with or without tidal breathing)
Predicted value sets
Predicted area or line display
Display default
Incentive display type
Printed graphs
Best test criteria
Interpretation and Lung Age indication
Dyspnea score and smoking status
Daily calibration reminder
Manual temperature adjustment
Indices selection
MVV options allow you to configure the following:
Choice of predicted values
Display ambient temperature during MVV test
Include graph of MVV maneuver in the final printout
Spirometry Measurements:
Relaxed Expiratory Vital Capacity (VC)
Forced Expired Volume in 0.75 seconds (FEV.75)
Forced Expired Volume in 1 second (FEV1)
Forced Expired Volume in 3 second (FEV3)
Forced Expired Volume in 6 seconds (FEV6)
Forced Vital Capacity (FVC)
Peak Expiratory Flow Rate (PEF)
FEV0.75 as a percentage of VC (FEV.75/VC)
FEV0.75 as a percentage of FVC (FEV.75/FVC)
FEV1 as a percentage of VC (FEV1/VC)
FEV1 as a percentage of FVC (FEV1/FVC)
FEV3 as a percentage of VC (FEV3/VC)
FEV3 as a percentage of FVC (FEV3/FVC)
FEV0.75 as a percentage of FEV6 (FEV.75/FEV6)
FEV1 as a percentage of FEV6 (FEV1/FEV6)
Maximum Expired Flow at 75% of FVC remaining (MEF75)
Maximum Expired Flow at 50% of FVC remaining (MEF50)
Maximum Expired Flow at 25% of FVC remaining (MEF25)
Mean Mid-Expiratory Flow Rate (MMEF)
Forced expiratory flow at 50% of volume as a percentage of VC (FEF50/VC)
Forced expiratory flow at 50% of volume as a percentage of FVC (FEF50/FVC)
Maximal voluntary ventilation indicated (MVV(ind))
Forced inspired volume in 1 second (FIV1)
Forced inspiratory Vital Capacity (FIVC)
Peak Inspiratory Flow Rate (PIF)
FIV1 as a percentage of FIVC (FIV1/FIVC)
Forced inspiratory flow at 25% of inhaled volume (FIF25)
Forced inspiratory flow at 50% of inhaled volume (FIF50)
Forced inspiratory flow at 75% of inhaled volume (FIF75)
Forced expiratory flow at 50% of volume as a percentage of FIF50 (FEF50/FIF50)
The time taken between 25% and 75% of the forced expired volume (MET2575)
Forced Expiratory Time (FET)
Tidal Volume (TV)
Expiratory reserve volume (ERV)
Inspiratory reserve volume (IRV)
Inspiratory capacity (IC)
Expiratory Relaxed vital capacity (EVC)
Inspiratory vital capacity (IVC)
Breathing frequency rate (FR)
Inspiratory time (Ti)
Expiratory time (Te)
Ti as a % of total breath time (Ti/Ttot)
Tidal volume as a % of Ti (TV/Ti)
Breath Rate BR
Breathing Time B.T
Volume Tidal VT
Expiratory Time – average time of expiration per Te
breaths in seconds
Inspiratory Time – average time of inspiration per Ti
breath in seconds
Total Tidal Breath Time in Seconds TTOT=Ti + Te
Ratio of Average Expiratory and Inspiratory Breaths Ti/Te
Average Time of Expiration per Breath as a ratio to Ti/TTOT
The Total Tidal Breath Time
Tests per subject: 5 VC maneuver
8 FVC maneuvers
Predicted Values: Various – depends upon national preference
Transducer: Micro Medical Bi-Directional Digital Volume.
Resolution: 10ml volume 0.03l/s flow
Accuracy: +/-3%. To ATS recommendations –
Standardization of spirometry 1994 update for flows and volumes
General Specifications:
Storage : >2000 tests including Flow/Volume loops and
Volume/Time curves
Printer Output: PLC3 compatible Hewlett Packard USB printers.
Display: Color 1/4VGA LCD.
Power supply: Input 100 to 240V, 50 to 60Hz.
Output 5V 2.0A (Class 1)
Type:MENB1010A0500F02
Battery Pack: Rechargeable Lithium Ion Polymer 3.7V 1600mA-hours.
Battery Life: Approximately 30 hours with a fully charged new battery
Dimensions: 4.7” x 3.1” x <1” - Transducer 2” x 2.4” x 3.5”
Weight: Unit: 7.1 ounces
Operating Temperature: 32 to 104 degrees Fahrenheit
Operating Humidity: 30% to 90% RH
Transport and Storage Temperature: -4 to 158 degrees Fahrenheit
Transport and Storage Humidity: 10% to 90% RH
A second Micro Loop distributed by Carefusion is also available. It comes in the Carefusion carrying case with software, a manual and all original accessories as well as a new battery and FREE SHIPPING.
US customers pay only what is paid on checkout. No US or Canadian taxes or border related fees like tariffs will be applied to this item on purchase or when it is delivered.
This item is for sale within Canada to appropriately qualified medical professionals and technicians employed in the repair and servicing of therapeutic and/or diagnostic respiratory devices. It will only be shipped to a Respiratory Therapist, MD or Bio-Medical Technician or other appropriately certified professional within Canada. These professionals can then use or distribute it at their discretion. Canadian buyers will be required to make payment in Canadian funds and to pay tax at 5% (GST only). Please contact the seller before purchase if you are in Canada.
Overseas shipping will be at cost with Payment by wire transfer or PayPal
*************************************************
DISCLAIMER:
Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The buyer is purchasing an item(s) which may have been used for medical purposes and may have the potential to again be used for this purpose, however, unless otherwise specified, at the time of sale the item is not considered to be bio-medically certified for use in any jurisdiction. The purchased item(s) is simply a device which operates in the limited manner described in the listing and/or electronic and mechanical components.
The purchaser agrees that the seller shall not be held responsible or liable:
* for any injuries or damages, whether incidental or consequential, associated in any way with the equipment;
* for compliance with regulations on ownership or use of the purchased item within the jurisdiction in which the purchaser resides and/or uses the item.
The purchaser, by buying this equipment, indicates their acknowledgement of, and agreement to the terms of this disclaimer.
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and other national/provincial/territorial/state and local regulatory agencies. If so, do not buy this item unless you are an authorized purchaser. If the item is subject to FDA regulation and it is to be used for regulated medical services in the USA, your payment for it indicates you are an authorized purchaser and will comply with these regulations. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA or the regulatory authorities in the jurisdiction in which the item is to be used
Return Policy
7 Day Return Policy. Customer must pay the return shipping costs when returning a product. If you find the item is not as described in the ad it may be returned for a purchase price refund. Generally, all of our items have an inspection warranty which kicks in upon delivery for a period of 7 to 14 days. It is not an operational warranty as most items sold on DOTmed require biomedical certification before use. If you require additional coverage please contact us prior to purchase.













 Get a CPS 2-Year Protection Plan starting at $5.99. Sold separately. Learn more
Get a CPS 2-Year Protection Plan starting at $5.99. Sold separately. Learn more