Toshiba open-bore 3-T MRI gets FDA nod
by Brendon Nafziger, DOTmed News Associate Editor | August 08, 2011

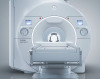
Vantage Titan 3-T
(Credit: Toshiba)
(Credit: Toshiba)
Toshiba America Medical Systems Inc. said Monday its open-bore 3-Tesla MRI received Food and Drug Administration 510(k) clearance.
The Vantage Titan 3-T features a 71-centimeter opening and the company's Pianissimo noise-reduction technology, which Toshiba claims quiets the 3-T MRI's notorious roar by up to 90 percent.
The Tustin, Calif.-based company says quieter exams help relax patients, so they move around less and the resulting image is less marred by motion artifacts.

 "From listening to our customers, we understand that patient compliance is one of the major issues impacting patient throughput and exam efficiency in the MR industry today," Stuart Clarkson, director of Toshiba's MR business unit, said in a statement.
"From listening to our customers, we understand that patient compliance is one of the major issues impacting patient throughput and exam efficiency in the MR industry today," Stuart Clarkson, director of Toshiba's MR business unit, said in a statement.
The Vantage Titan also offers non-contrast magnetic resonance angiography technologies for examining blood vessels without using contrast agents. And it uses Toshiba's Atlas Speeder coils, which are integrated into the exam table, to reduce scan times.
The Vantage Titan 3-T features a 71-centimeter opening and the company's Pianissimo noise-reduction technology, which Toshiba claims quiets the 3-T MRI's notorious roar by up to 90 percent.
The Tustin, Calif.-based company says quieter exams help relax patients, so they move around less and the resulting image is less marred by motion artifacts.
New Fully Configured 80-slice CT in 2 weeks with Software Upgrades for Life
For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.

The Vantage Titan also offers non-contrast magnetic resonance angiography technologies for examining blood vessels without using contrast agents. And it uses Toshiba's Atlas Speeder coils, which are integrated into the exam table, to reduce scan times.
You Must Be Logged In To Post A CommentRegisterRegistration is Free and Easy. Enjoy the benefits of The World's Leading New & Used Medical Equipment Marketplace. Register Now! |
|