by
Lauren Dubinsky, Senior Reporter | November 14, 2017
Call to action for physicians
The American Heart Association, the American College of Cardiology and the Heart Rhythm Society issued updated guidelines on Wednesday that recommend the use of subcutaneous implantable cardioverter-defibrillators to treat patients with ventricular arrhythmia.
“The pace of technologic innovation and the accumulation of new clinical evidence is remarkably rapid in this field,” Dr. Kenneth Stein, senior VP and CMO of rhythm management and global health policy at Boston Scientific, told HCB News. “The revised recommendations incorporate new clinical data and real-world evidence gathered since the prior guideline publication in 2006.”
That includes findings from the EFFORTLESS U.S. postmarket approval study published in the August issue of the
Journal of the American College of Cardiology and a recent meta-analysis published in September in
JACC: Clinical Electrophysiology.



Ad Statistics
Times Displayed: 47202
Times Visited: 1431 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
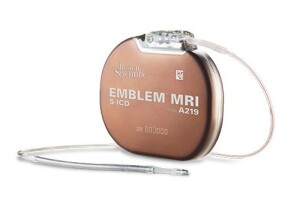
Boston Scientific’s EMBLEM MRI S-ICD System is currently the only S-ICD on the market and the only implantable defibrillator that protects patients at risk of sudden cardiac death without touching the heart.
The new guidelines support the use of an S-ICD device for all patients who meet the criteria for an ICD without a need for pacing. The guidelines also strongly recommend the use of an S-ICD device as the standard of care for patients who have inadequate vascular access or are at high risk for infection including those with diabetes.
As is the case with any new therapy, physicians seek procedural experience and long-term clinical data before recommending a newer device for patients who could otherwise be treated with the prior standard of care. Stein views these recommendations as a call to action for physicians to include the S-ICD System when making treatment decisions with their ICD-indicated patients.
“The new guidelines, coupled with the most recent long-term clinical and real-world outcome data, provide further confirmation that the S-ICD System can and should be considered as a first-line therapy for those ICD-indicated patients without a pacing indication,” he added.

