by
John R. Fischer, Senior Reporter | October 18, 2017
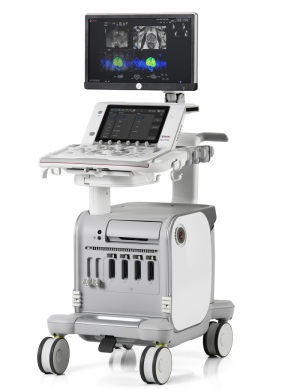
Esaote unveiled its flagship ultrasound system, MyLab9 eXP, this past weekend at the Journées Francophones de Radiologie (JFR 2017), hosted by the Société Française de Radiologie in Paris.
The Italian-designed system offers hospitals and clinics a variety of features for enhancing image clarity, workflow and performance to ensure greater confidence in diagnoses, enhanced patient throughput and the creation of better-informed health care systems.
“One feature is about the clarity in terms of image quality,” Massimo Rosa, chief marketing officer at Esaote, told HCB News. “The second one is the workflow, in terms of better user interface to improve daily use. The third one is the performance in terms of time, in terms of response of the system, in terms of better usability in day-to-day hospital use. We can say that we have three pillars of benefits for this system.”



Ad Statistics
Times Displayed: 56950
Times Visited: 1656 Ampronix, a Top Master Distributor for Sony Medical, provides Sales, Service & Exchanges for Sony Surgical Displays, Printers, & More. Rely on Us for Expert Support Tailored to Your Needs. Email info@ampronix.com or Call 949-273-8000 for Premier Pricing.
MyLab9 uses non-composite single-crystal probe technology to deliver high image clarity. It also is equipped with a floating keyboard, a tablet-like touchscreen, a simple control panel and a full HD wide-format screen for ease-of-use along with a solid-state hard disk (SSD), an Intel Core i7 processor, and Windows 10 for data security and power processing requirements.
The system provides a multi-modality approach that allows for fast access to other imaging modalities and PACS systems for immediate clinical follow-up and fusion imaging. It is ergonomic, offering image optimization tools such as the easyMode touch tool, which enables physicians to focus on patients during procedures without distractions or experiencing complex technical routines within the system.
Other features include smart upgradability, remote serviceability, long-term maintenance options and transducer compatibility.
Massimo Rosa, chief marketing officer at Esaote, told HCB News that the system has the potential to become a standard tool among physicians.
“This is our expectation, because we try to give the best solution for the physician in terms of easy usability,” he said. "We want to address the majority of needs of the physician because we think that with the automatic tools, it will be easier for them to perform the measure. This is our contribution in terms of position."
The system will be on display in November at the Radiological Society of North America conference in Chicago. It is CE-marked and currently FDA-pending. Esaote expects to receive approval for the system in the U.S. in the second half of 2018.