by
John R. Fischer, Senior Reporter | June 01, 2023
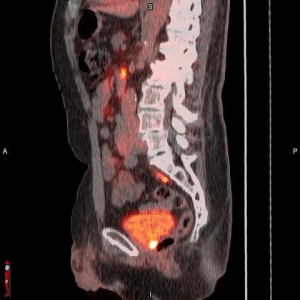
The FDA has cleared Blue Earth Diagnostics' POSLUMA injection for PET imaging in prostate cancer patients.
The FDA has given Blue Earth Diagnostics the green light for its POSLUMA (Flotufolastat F 18) injection, the first radiohybrid PSMA (prostate-specific membrane antigen)-targeted PET imaging agent for prostate cancer.
The agent is designed for men with suspected prostate cancer metastasis who are eligible for initial definitive therapy, such as surgery or radiotherapy, and men with suspected recurrent cancer based on elevated serum prostate-specific antigen levels.
Through its distributor, PETNET Solutions, a Siemens Healthineers Company, Blue Earth Diagnostics will distribute it starting in early June through certain radiopharmacies in PETNET Solutions’ national radiopharmacy network, followed by others across the country in the coming months.



Ad Statistics
Times Displayed: 656
Times Visited: 5 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
The decision follows positive results from two Phase 3 trials, LIGHTHOUSE and SPOTLIGHT, in which POSLUMA provided valuable clinical information prior to surgery or radiotherapy that would likely change how these patients are managed.
“The highly variable nature of recurrent prostate cancer presents clinical challenges, and up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years,” said Dr. David M. Schuster, professor of radiology and imaging sciences at Emory University School of Medicine, and coordinating investigator for the SPOTLIGHT study.
In the LIGHTHOUSE study, for patients who were candidates for initial definitive therapy, POSLUMA detected pelvic lymph nodes with overall 96% specificity compared to the histopathology standard of truth, in patients at intermediate, high and very high risk who were scheduled to undergo radical prostatectomies and pelvic lymph node dissection. Its overall sensitivity was 24%, reported MPR, a publication under Haymarket Medical Network. In the SPOTLIGHT study, which included patients who underwent prior therapy and tested POSLUMA’s potential as a decision-making tool, the agent detected the location and extent of suspected prostate cancer recurrence based on elevated PSA, even when levels were low, with overall 83% success.
“Up to 25% of patients with primary prostate cancer may have detectable regional pelvic lymph node metastases, which are correlated with a risk for recurrence and associated overall survival,” said Dr. Brian Chapin, associate professor in the department of urology and division of surgery at the University of Texas MD Anderson Cancer Center, in a statement.
In testing its safety, clinical trials showed adverse effects in ≥0.4% of 747 patients with initial or recurrent cancer that included diarrhea, blood pressure increases, and injection site pain.
Because it is based on novel radiohybrid technology, POSLUMA also offers therapeutic potential, making it a candidate for the growing field of theranostics.

