by
Brendon Nafziger, DOTmed News Associate Editor | March 04, 2011
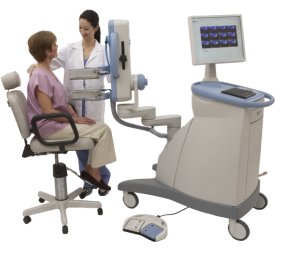
Naviscan Inc. this week announced the European launch of its positron emission mammography scanner and said it would share preliminary data on using PEM for lymph node staging for patients with breast cancer.
The San Diego-based, 16-year-old device manufacturer said that its PEM scanners, which have been commercially available in the United States for four years, would be sold in Europe by Q2.
The company also said it would share early results from a study conducted by University of Chicago researchers about using the PEM to help stage breast cancer at the European College of Radiology's 2011 annual meeting, which runs through Monday in Vienna.



Ad Statistics
Times Displayed: 2839
Times Visited: 27 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
The prospective study, which involved 20 newly diagnosed breast cancer patients, had women undergo clinical examinations and ultrasound and MRI scans for axillary lymph node staging. The findings were then compared with pathological analysis of the nodes.
Naviscan said the results, still preliminary, suggest PEM has 88 percent sensitivity and a negative predictive value of 91 percent in assessing ALN status.
"[T]hese findings, in conjunction with PEM's more extensively documented role in evaluating the breasts themselves as well as whole-body PET's excellent whole body staging, paves the way for a complete breast cancer work-up-primary tumor, regional and distant metastases--with one [fluorodeoxyglucose] dose," Dr. Daniel Appelbaum, associate professor of radiology from the University of Chicago Medical Center, said in a statement.