by
Olga Deshchenko, DOTmed News Reporter | March 18, 2011
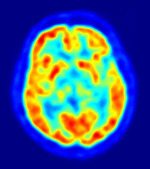
The U.S. Food and Drug Administration rejected Amyvid, a molecular imaging agent for the detection of Alzheimer's disease, its makers said Friday.
Amyvid, a PET imaging agent under investigation for the detection of beta-amyloid plaque in living patients' brains, is produced by Eli Lilly and Company's subsidiary Avid Radiopharmaceuticals, Inc.
In its denial of approval for the dye, the FDA focused "on the need to establish a reader training program for market implementation that helps to ensure reader accuracy and consistency of interpretations of existing Amyvid scans," the drug's makers said in a statement.



Ad Statistics
Times Displayed: 2157
Times Visited: 29 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
In January, an FDA advisory panel
asked the company for more evidence on the florbetapir F18 agent. The companies said they've been working on the issues raised by the panel and are continuing to cooperate with the FDA.
"Lilly and Avid have been engaged in an active and ongoing dialogue with the FDA," said Wei-Li Shao, Lilly brand director for the agent. "We remain confident in the data submission package for Amyvid."