by
Olga Deshchenko, DOTmed News Reporter | March 30, 2011
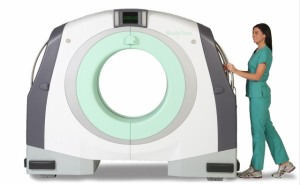
Neurologica Corp. said Wednesday it received U.S. Food and Drug Administration approval for its BodyTom, a pioneering portable, full body multi-slice CT scanner.
The scanner has an 85-centimeter gantry and a 60-centimeter field of view. Similar to a portable chest X-ray system, the BodyTom can be moved from room to room and used in a variety of settings, including intensive care units, emergency departments and operating rooms, according to the company.
[Read about the growing trend of CT in the ER and OR]



Ad Statistics
Times Displayed: 1227
Times Visited: 6 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
"Our goal with the BodyTom is to deliver high quality CT imaging in a completely portable package that is capable of scanning an entire patient's body," Dr. Colin McDonald, chief medical officer of NeuroLogica, said in prepared remarks.
The Danvers, Mass.-based company also manufactures the CereTom, a small bore, 8-slice portable CT scanner for brain perfusion and brain angiography.
The BodyTom is commercially available this year, the company said.