by
Brendon Nafziger, DOTmed News Associate Editor | August 06, 2012
Ion Beam Applications S.A. spinoff IBA Molecular said Monday it struck an exclusive deal with Piramal Imaging to manufacture and distribute 18F-Florbetaben, an investigational PET imaging agent that could help doctors measure beta-amyloid plaque deposits in living patients with suspected Alzheimer's disease. Terms of the deal weren't disclosed.
Under the agreement, IBA Molecular gains exclusive distribution rights for the drug in Europe and the United States, and can start shipping it once it becomes approved by regulators. The drug has not yet been cleared for sale in either market.
Thomas Matthes, product general manager with IBA Molecular, told DOTmed News that the groups plan to file a submission for 18F-Florbetaben with the U.S. Food and Drug Administration by the end of the year, followed closely by a European application. The FDA application would take at least 12 months, Matthes said.



Ad Statistics
Times Displayed: 137968
Times Visited: 7965 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
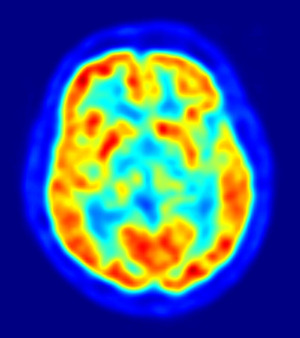
Piramal released phase III results for the drug in April at a meeting of the American Academy of Neurology, which will serve as the basis of the FDA filing. By overlaying MRI and 18F-Florbetaben PET images on top of each other, doctors were able to achieve 77 percent sensitivity and 92 percent specificity in finding beta-amyloid deposits in six brain regions. For routine clinical practice, Piramal reported its assessment protocols achieved 100 percent sensitivity and 92 percent specificity with strong inter-reader agreement.
Mumbai-based Piramal bought the rights for 18F-Florbetaban from drug giant Bayer AG in April.
Once released, 18F-Florbetaben will go up against rival PET beta-amyloid imaging agents, including Eli Lilly & Co.'s Amyvid,
approved by the FDA in April, and GE Healthcare's flutemetamol. GE also expects to
submit an application for its drug with U.S. regulators by the end of the year.
IBA Molecular runs 54 PET isotope facilities in Europe, North American and Asia. Its U.S. headquarters are in Dulles, Va.