by
Brendon Nafziger, DOTmed News Associate Editor | August 17, 2012
Siemens Healthcare said Thursday that the Food and Drug Administration has cleared software that helps quantify amyloid plaque deposits on PET scans in patients at risk for Alzheimer's.
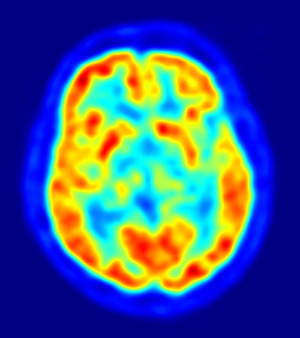
Called syngo.PET Amyloid Plaque, the program can help automatically quantify the uptake of Amyvid (florbetapir), the first PET imaging agent approved by the FDA to measure beta-amyloid plaques in the brains of living patients. The drug, manufactured by Eli Lilly & Co., received FDA approval in April. Although it can't really allow doctors to make a final diagnosis for Alzheimer's, it could help them rule out the disease for patients suffering cognitive impairment.
Syngo.PET registers a brain scan against a reference model of a known PET amyloid brain, comparing uptake ratios along six regions of interest in the cortex, Siemens said. Siemens and Avid Pharmaceuticals, the original developer of Amyvid and now an Eli Lilly subsidiary, presented a study on the technology based on scans from 210 patients at SNM's annual meeting in June.



Ad Statistics
Times Displayed: 1727
Times Visited: 9 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
The software also works with Siemens' new mBiograph PET-CT scanner, which was released in February.
Siemens also supplies Amyvid through its PETNET subsidiary, the biggest chain of radiopharmacies in the United States.