by
Loren Bonner, DOTmed News Online Editor | March 26, 2014
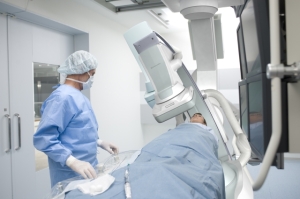
Shimadzu's Trinias Ceiling Mounted
(C12) Crossover Angiographic System
Shimadzu Medical Systems USA announced on Tuesday that the U.S. Food and Drug Administration granted 510(k) clearance for the company’s new digital angiography family, comprising three systems: the C12 ceiling-mounted system, the F12 floor-mounted system and a new biplane system.
The company said it’s the most important new medical modality that Shimadzu has introduced in the U.S. and globally since 2007.
Shimadzu is marketing the system for improved workflow, lower radiation dose and ergonomics.



Ad Statistics
Times Displayed: 2080
Times Visited: 9 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
All systems incorporate Shimadzu’s ultra-high speed SCORE PRO image processing technology, which Shimadzu said enhances fluoroscopic images while maintaining ALARA (As Low As Reasonably Achievable) dose considerations for all clinical staff.
As an angiographic interventional system and cardiac interventional system, the systems also provide 3-D application techniques.

