by
Loren Bonner, DOTmed News Online Editor | April 23, 2014
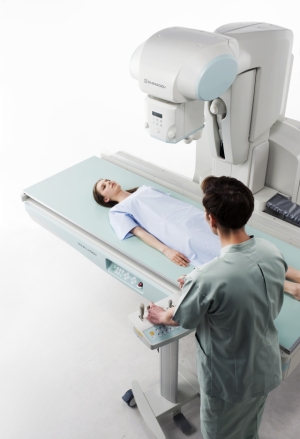
Shimadzu's new R/F table system
Shimadzu Medical USA announced on Tuesday that the U.S. Food and Drug Administration has granted clearance for its new R/F system, called the Sonialvision G4 universal digital R/F table system.
The new generation system includes some features such as a table that can support a patient weighing up to 700 pounds, and it can examine patients in wheelchairs/stretchers with its extended imaging chain.
The company is marketing the system for a wide variety of examinations including angiography, endoscopy, video fluoroscopy for gastrointestinal series and swallowing exams, orthopedic exams, and general radiography.



Ad Statistics
Times Displayed: 797
Times Visited: 5 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
This release from Shimadzu follows on the heels of another major product
announcement from the company a few weeks ago, the Trinias digital angiography system.

