by
Lisa Chamoff, Contributing Reporter | June 19, 2014
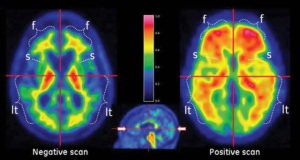
Vizamyl image courtesy
of GE Healthcare
From the June 2014 issue of HealthCare Business News magazine
Backers of PET amyloid imaging were dealt a blow last July when the Centers for Medicare and Medicaid Services said there was insufficient evidence to conclude that Amyvid, the radiotracer manufactured by Eli Lilly and Company that detects amyloid plaque in the brain, improves health outcomes for Medicare beneficiaries with Alzheimer’s and related dementia.
Despite the hurdle in front of full coverage for scans that are considered a gateway to an Alzheimer’s diagnosis, the future of the radiopharmaceutical industry still looks bright.
Officials at Eli Lilly and Co. say that the CMS decision — which allows for reimbursement of one Amyvid PET scan to rule out Alzheimer’s disease, but only for patients enrolled in clinical trials under what is known as a coverage with evidence development program — will not change its commitment to innovation in the field of Alzheimer’s disease.



Ad Statistics
Times Displayed: 129612
Times Visited: 7359 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
“However, we acknowledge that such uncertainty in the reimbursement process creates a strong disincentive for future investments in research and development,” says Stefanie Prodouz, a spokeswoman for Lilly Bio-Medicines. “To foster future innovation, manufacturers need predictable pathways to coverage.”
Despite the unpredictable pathways, companies are moving forward. In March, the U.S. Food and Drug Administration approved a third Alzheimer’s PET agent, Piramal Imaging’s Neuraceq. GE Healthcare’s Vizamyl was approved last year, while Amyvid was approved in 2012.
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) still believes that there are sufficient benefits and evidence to support immediate coverage, but has directed its efforts toward proposing a process for a coverage with evidence development program.
SNMMI has been working together with the Alzheimer’s Association to develop the clinical trial that CMS has deemed necessary to reconcile the evidentiary gaps they outlined in the final coverage decision for beta-amyloid imaging agents.
“The problem is the CMS wants evidence of positive patient outcomes,” says SNMMI President Dr. Gary Dillehay. “That’s difficult in a disease like this that’s progressive.”
Dr. Dean Hartley, director of science initiatives at the Alzheimer’s Association, who was previously an associate professor in the neurological sciences department at Rush University Medical Center in Chicago, says the process is ongoing and that the groups have had direct discussions with CMS to better understand the issues involved.

