by
Lauren Dubinsky, Senior Reporter | October 10, 2016
Clinical research demonstrates
its benefits
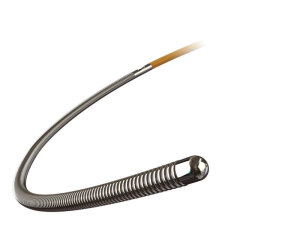
St. Jude Medical announced today that the FDA has cleared its new PressureWire X Guidewire fractional flow reserve (FFR) measurement system. The improved design and shape retention of the system’s tip enables cardiologists to perform FFR measurement in patients with complex anatomies.
Coronary artery disease (CAD) patients undergo percutaneous coronary intervention (PCI) procedures to open coronary blood flow blockages and restore blood flow to their hearts. The PressureWire Guidewire FFR measurements are used by the cardiologists to determine the severity of narrowings in the coronary arteries.
Even though those measurements can lead to more accurate diagnoses and better treatment decisions, only one in five of the 900,000 PCI procedures performed per year in the U.S. use FFR guidance. St. Jude Medical is trying to change that with clinical research.



Ad Statistics
Times Displayed: 7172
Times Visited: 104 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
“For years, clinical research has confirmed that fractional flow reserve is one of the most important tools available when assessing coronary lesions and making informed treatment decisions during percutaneous coronary intervention,” Dr. Annapoorna Kini, the director of the cardiac cath lab and of the interventional fellowship program at Mount Sinai Medical Center, said in a statement.
Along with the latest PressureWire Guidewire, the company launched the PRESSUREwire REGISTRY. It’s a multi-center clinical trial that’s going to test the routine use of FFR measurement and clinical outcomes of FFR-guided PCI in patients with acute coronary syndrome.
This trial will expand on other clinical trials that have been designed to evaluate FFR technology, including the FAME (Fractional Flow Reserve vs. Angiography in Multivessel Evaluation) trial, which is sponsored by St. Jude Medical.
Those trials found that the PressureWire Guidewire technology can improve patient outcomes, compared to angiography alone, in patients with CAD. They also showed that the technology reduces the risk of death or heart attack for patients undergoing PCI, and cuts health care costs.
Two-year data from the FAME 2 trial demonstrated that FFR-guided PCI combined with medical therapy reduced revascularization by 77 percent, compared to medical therapy alone, in patients with at least one significant coronary blockage with a FFR value of at least .80.
Five-year data from the FAME study that was released in 2015 confirmed the sustained, long-term benefits of FFR-guided PCI over angiography-guided intervention.
Back to HCB News

