by
John R. Fischer, Senior Reporter | October 24, 2017
Following FDA approval, Kent Imaging’s perfusion imaging tool, KD203 is now available for purchase in the U.S.
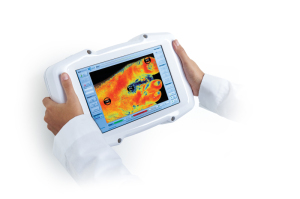
The Calgary-based company’s multispectral medical imaging device enables accurate diagnosis, improved treatment planning and efficient patient monitoring by providing physicians with insight into the healing abilities of tissue by directly showing the oxygenation of blood in tissue.
“Kent's KD203 is unique, in that its patented technology offers value-based tissue oxygenation levels (StO2),” Don Chapman, executive chairman at Kent Imaging, told HCB News. “As a low-cost, easy-to-use imaging device, it is completely noninvasive, eliminating the need for contact or injected dyes (required by traditional technologies) and instantly capturing diagnostic insight into the viability of tissue in many applications.”



Ad Statistics
Times Displayed: 2188
Times Visited: 31 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
Perfusion imaging traditionally requires the use of dyes and injections, making the process more time-consuming and invasive. The monitoring of the movement of dye provides only an indirect measurement of blood oxygen levels in tissue. KD203 measures oxygen level in tissue directly and is noninvasive.
The portable, hand-held device can collect data in less than a second using a variety of health applications, to provide physicians with simplified diagnostic readings and insights for analysis on the best course of treatment before, during and after clinical and surgical care.
It requires only minimal training, offers accurate and unlimited imaging and storage, and is low-cost, with no disposables. It also has a battery life of more than eight hours and has been applied to wound care, breast reconstruction, bowel resections and cardiac bypass surgeries.
“Its many applications, low cost and ease of use give this device the ability to be used throughout hospitals from the ER to the surgical suites, to the wards, becoming a standard tool among providers and physicians,” he said.
The
Society of Nuclear Medicine and Molecular Imaging (SNMMI) recently published a set of criteria for the use of ventilation/perfusion imaging in pulmonary embolism.
KD203 has obtained Class II FDA clearance and is also available for sale in Canada.