by
John R. Fischer, Senior Reporter | February 14, 2018
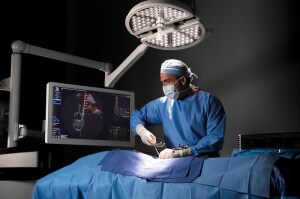
7D Surgical's Machine-vision Image
Guided Surgery system
7D Surgical, a manufacturer of advanced optical technologies and machine vision-based registration algorithms, is closer to commercially launching its Machine-vision Image-Guided Surgery (MvIGS) system with the completion of its largest round of financing for the product.
The Toronto-based company plans to distribute the 510(k) solution to assist surgeons in performing spinal registration in less time and at lower costs, while reducing radiation exposure and improving efficiency and safety.
"The latest investment into 7D Surgical will allow our team to aggressively scale our commercial footprint to meet the demand for our MvIGS technologies," Beau Standish, chief executive officer at 7D Surgical, said in a statement. "We believe the hospital market will continue to adopt innovations that will lower costs while improving efficiency and safety. The 7D Surgical system is perfectly positioned to fulfill each of these objectives."




Ad Statistics
Times Displayed: 45175
Times Visited: 1385 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
The system consists of integrated principles found in self-driving vehicles and facial recognition, and saves operational time by enabling surgeons to perform spinal registration in seconds through 7D Surgical's Flash Registration and Flash Fix technology.
Patient registration can be updated at any time by clicking down on a foot pedal. Exposure to intraoperative radiation is also reduced for both patients and staff members with the MvIGS emitting visible light.
"We have been thrilled by the response from clinicians who have implemented the MvIGS technology," Brian Stuart, vice president of sales and marketing for 7D Surgical, said in a statement. "The immediate and positive impact on surgical workflows, radiation reduction and sterile control of the technology has strongly resonated with surgeons and operating room staff. These advantages, along with the significant cost savings over current options, make MvIGS a win for everyone, including hospital administration."
The system can currently be viewed through a state-of-the-art audio-visual experience and will be demonstrated live at the Texas Association of Neurological Surgeons Meeting in Houston from Feb. 9-10 and at the Spine Summit Meeting in Orlando from March 15-16.
The solution
was cleared by the FDA and received a medical device license from Health Canada for spine procedures last January.
7D Surgical did not respond for comment.

