by
Barbara Kram, Editor | August 14, 2007
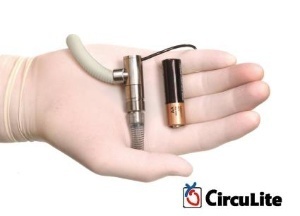
Synergy™ Pocket Circulatory
Assist Device
HACKENSACK, N.J. and AACHEN, Germany (BusinessWire EON) -- CircuLite™, Inc. has reported the launch of the Company's clinical development program for its Synergy™ Pocket Circulatory Assist Device with the successful implantation of the first patient in a European feasibility trial. Synergy is a miniature implantable blood pump, the size of a AA battery, that can be implanted superficially in a pocket, like a pacemaker. The device is designed to provide long-term, partial circulatory support in patients with chronic heart failure. The primary objective of the first-in-man trial is to assess the safety of the device in patients with chronic heart failure who are waiting to receive heart transplants. Bart Meyns, M.D., Ph.D., Professor and Chief of Cardiac Surgery at Gasthuisberg University Hospital (Katholieke Universiteit) in Leuven, Belgium, performed the first implant.
"The first implant of the Synergy device was very successful and the patient has already been discharged home and is doing very well," said Prof. Meyns, Principal Investigator of the trial. "While CircuLite's feasibility clinical study is in a bridge-to-transplant setting, the ultimate need for this type of device will be among those chronic heart failure patients who may not be eligible for a heart transplant. A partial support approach to chronic heart failure treatment may be better able to address the treatment needs and improve the quality of life for this unserved group of patients, who otherwise have no other options."
News Image The CircuLite technology is designed to provide a new treatment option for over two million chronic heart failure patients worldwide who continue to be significantly symptomatic despite appropriate, optimal medical and device-based therapies. While feasibility studies will examine the hemodynamic and clinical effects of the Synergy device in patients that are awaiting heart transplants, CircuLite's ultimate goal is to expand the treatment of heart failure to the chronic, ambulatory patient in order to improve their quality of life by giving them an elective, less-invasive option to increase blood flow from the heart.



Ad Statistics
Times Displayed: 1943
Times Visited: 9 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
"The first-in-man implant of our Synergy device is a significant milestone not only for our company, but for the chronic heart failure community as a whole, especially patients, who we hope will someday benefit from this potentially life-changing therapy," said Paul Southworth, President and CEO of CircuLite. "CircuLite is striving to transform the treatment model for this condition to long-term, partial circulatory support. The commencement of this trial is an achievement that brings together over 11 years of engineering and research and demonstrates our leadership position in this space. This trial will provide us with important data to support our additional planned studies, including a CE Mark trial in Europe, as well as our Investigational Device Exemption (IDE) trials in the United States."

