by
Brendon Nafziger, DOTmed News Associate Editor | February 01, 2011
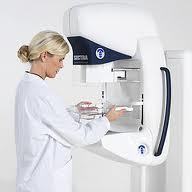
Swedish medical device company Sectra Medical Systems said Tuesday it received Canadian approval to market its low dose digital mammography device, an important milestone on its way to U.S. clearance.
The Sectra MicroDose Mammography uses a technology called photon counting which digitally counts X-ray photons that strike the detector. This method exposes patients to half the radiation dose of other digital mammo methods, according to materials supplied by the company.
The device is already available in 18 European countries, Australia and New Zealand, the Linköping, Sweden-based company said. It's pending U.S. Food and Drug Administration clearance.



Ad Statistics
Times Displayed: 656
Times Visited: 5 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
"The next step is the FDA approval that would mean that mammography clinics throughout the U.S. will also have the possibility to offer women mammography examinations with the lowest possible dose," Jesper Söderqvist, head of Sectra's mammography group, said in a statement.
In a white paper released last week, Sectra said the adoption of its units in Sweden from 2006 to the present caused the population dose from radiation in mammography to fall by around 12 percent.
According to the study, in 2008, the average screening dose for a single exposure from Sectra's system was 0.48 mGy, whereas from most other digital systems it was 0.97 mGy. For film screening, the average dose was about 1 mGy.