by
Brendon Nafziger, DOTmed News Associate Editor | March 29, 2011
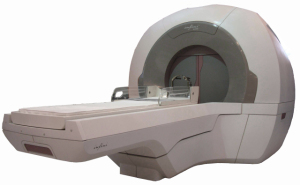
The Infini gamma ray
neurosurgery system
MASEP Infini said it received U.S. Food and Drug Administration 510(k) clearance last week for its new stereotactic neurosurgery device -- a lower-priced system the decade-old company hopes will help drive international sales.
The Infini gamma ray neurosurgery system features automated collimator exchange and 3-D patient positioning, and allows for precise, powerful dose-sculpting to kill brain tumors.
But the City of Industry, Calif.-based company claims the Infini has lower purchase and maintenance costs, in part because it uses about one-sixth the cobalt sources of similar devices and has fewer collimator channels, even though the energy per kilogram delivered to the patient is the same thanks to the device's "rotary focus" design.



Ad Statistics
Times Displayed: 656
Times Visited: 5 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
"You can build these machines for less money than comparable gamma ray equipment," Bruce Meredith, vice president of clinical affairs and marketing with MASEP, told DOTmed News.
The rotating gamma ray technology was originally developed by engineers in China, and marketed by MASEP group's first branch in Shenzhen. The company's first intracranial radiosurgery platform, the semi-automatic MASEP Model SRRS, was released in 1999.
The company, whose full name is MASEP Infini Medical Science Technology Development Co. Ltd., received the FDA's clearance letter around March 23. MASEP is finishing up U.S. operations for the device, setting up a training center and a final test inspection site.
Currently, one Infini device is installed in China, and one's shipping to Turkey in about three to four weeks, Meredith said. It received CE marking in 2010, according to information on the company's website.
MASEP products are installed in about 40 sites in China and Vietnam, Meredith said.

