by
Brendon Nafziger, DOTmed News Associate Editor | August 04, 2011
Siemens Healthcare said Wednesday its hybrid PET-MR device received approval from Canada's health authorities, getting a Health Canada Medical Device License.
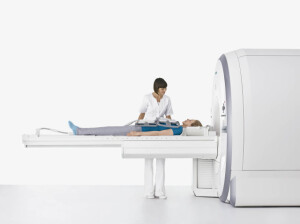
The device, the Biograph mMR, is the first fully integrated PET and MR scanner. It's already available in the U.S. and Europe, having received
FDA clearance and
CE marking in June.
Siemens said the new technology would likely have cancer, heart disease and neurological applications. Simultaneous acquisition of PET and MRI images also means it's faster than sequential staging with individual PET and MR scanners.



Ad Statistics
Times Displayed: 136626
Times Visited: 7924 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
The Biograph mMR has already netted a couple of awards. Frost & Sullivan gave it a prize earlier this year for new product innovation, and last month it got
a 2011 Red Dot award for product design, one of design's most prestigious awards.