by
Loren Bonner, DOTmed News Online Editor | January 08, 2013
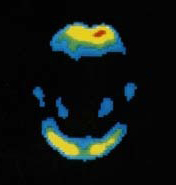
GE Healthcare is one step closer to getting its positron emission tomography (PET) imaging agent to market. Today, the company announced that the U.S. Food and Drug Administration has accepted its application to review flutemetamol, a PET amyloid imaging agent used in the visual detection of beta amyloid in the brains of patients being evaluated for Alzheimer's disease and related cognitive disorders. Additionally, the European regulatory body, the European Medicines Agency, will also consider GE's application for review.
In July 2012, GE released
results from a phase 3 study on flutemetamol, which formed the basis of the FDA application. In that study, flutemetamol was able to successfully measure beta-amyloid plaque in living patients.
Eli Lilly & Co.'s Amyvid received FDA approval in the spring of 2012. It's currently the only available PET imaging agent to measure beta-amyloid plaque in patients with suspected Alzheimer's disease.



Ad Statistics
Times Displayed: 1227
Times Visited: 6 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.