by
John R. Fischer, Senior Reporter | September 07, 2017
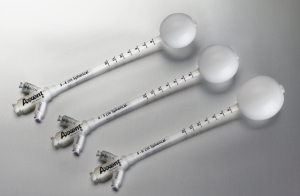
iCAD's Xoft Axxent balloon applicators
The China Food & Drug Administration (CFDA) has approved the use of iCAD’s Xoft Axxent balloon applicators for treating early-stage breast cancer.
Doctors and clinicians in China can now use the applicators, a component of the the Xoft Axxent Electronic Brachytherapy (eBx) System, to administer intraoperative radiation therapy (IORT) to the site of tumors during the lumpectomy for patients with stage one or two breast cancer as well as those with Ductal carcinoma in situ (DCIS) that has not yet progressed to the lymph nodes.
“More and more women are searching for effective alternatives for the treatment of early stage breast cancer,” Daniel Arnoff, vice president of global sales at iCAD, told HCB News. “Many women cannot comply with the six to eight weeks of external beam radiation following their surgery. IORT is done at the time of surgery, allowing appropriately selected patients to complete their full course of radiation in one day.”



Ad Statistics
Times Displayed: 79594
Times Visited: 2813 Ampronix, a Top Master Distributor for Sony Medical, provides Sales, Service & Exchanges for Sony Surgical Displays, Printers, & More. Rely on Us for Expert Support Tailored to Your Needs. Email info@ampronix.com or Call 949-273-8000 for Premier Pricing.
The Xoft System’s miniaturized x-ray source is inserted into an applicator as a channel for administering a single and exact, high-dose rate of low energy radiation treatment to the lumpectomy cavity. The applicator delivers the dose, targeting the cancer cells directly, so as to reduce exposure of healthy surrounding tissue to the radiation.
The applicators come in different sizes and shapes and can be filled with different volumes of saline to fit the contour of the cavity exactly and enable delivery of a more conformal dose of radiation. They also come in a sterile pack, eliminating the need for sterilization and decreasing the risk of cross-contamination.
Arnoff says the Xoft system and its applicators have the potential to become a standard option for treating breast cancer in China, and that with more research, could be used to treat other forms of cancer.
“As additional data emerge in support of other clinical applications, we expect that other countries, including China, will consider utilizing the Xoft System and its variety of applicators for a range of cancers,” he said.
The Xoft system and its applicators are FDA-approved and CE-marked to treat cancer in any part of the body, including early-stage breast cancer, non-melanoma skin cancer and gynecological cancers.

