by
Lauren Dubinsky, Senior Reporter | September 20, 2017
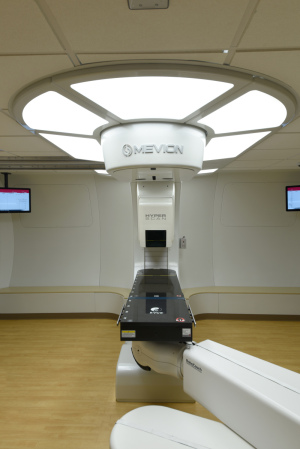
Mevion Medical Systems'
MEVION S250i
Mevion Medical Systems announced on Monday that it submitted its 510(k) Premarket Notification to the FDA for the MEVION S250i proton therapy system with HYPERSCAN technology.
"[This system] is a groundbreaking change to the way pencil-beam scanning proton therapy is delivered today," Joe Jachinowski, CEO of Mevion, told HCB News. "It enables faster, sharper, and more robust treatment delivery through a few key features."
The HYPERSCAN technology allows for faster energy layer switching and optimized spot sizes. It also features the Adaptive Aperture proton collimation system, which is the first commercial PBS multileaf collimator that's capable of multilayer conformal field delivery.



Ad Statistics
Times Displayed: 137680
Times Visited: 7952 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
"This collimating system can sharpen outer edges and internal gradients for any field — up to [three times] sharper field penumbras have been demonstrated," said Jachinowski. "This should enable greater performance, in particular, for shallow treatments such as head and neck or intracranial tumors."
A combination of technologies also work together to deliver the dose faster, which is particularly important for thoracic treatments where motion management is an issue. The target organ may move significantly when a patient is breathing, and current proton therapy systems can take several breaths to deliver a single field.
"HYPERSCAN technology will support scanning a lung tumor in a single breath hold," Jachinowski explained. "This should add confidence in treating these tumors."
The first MEVION S250i system has already been installed at MedStar Georgetown University Hospital in Washington, D.C., and is undergoing final testing. Once it receives FDA clearance, this will be the first in the world to treat patients using the HYPERSCAN technology.
The system will be on display at this year's American Society for Radiation Oncology annual meeting in San Diego, California next week.
"Given the growing evidence for proton therapy and expanded guidelines, we expect the HYPERSCAN technology to really resonate with clinicians and administrators," noted Jachinowski.
He added that it's possible that the MEVION S250i may receive FDA clearance in 90 days or less. But it's entirely dependent on the FDA and whether the agency has further questions.

