by
Lauren Dubinsky, Senior Reporter | November 16, 2017
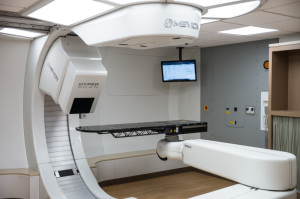
First European installation
to be at ZON PTC
Mevion Medical Systems announced on Monday that its MEVION S250i Proton Therapy System scored CE mark.
"With the continued demand for to access proton therapy in Europe and larger proton facilities falling into bankruptcy, the MEVION S250i has been receiving a great deal of interest," Michael Tajima, director of product management and marketing at Mevion, told HCB News.
The system comes with the company's latest generation of pencil-beam scanning technology called HYPERSCAN. It incorporates fast energy layer switching, optimized spot sizes and the Adaptive Aperture proton multi-leaf collimator system.




Ad Statistics
Times Displayed: 44187
Times Visited: 1363 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
HYPERSCAN was designed to deliver sharp radiation gradients to fully treat tumors, while it also reduces exposure to adjacent healthy tissue. It provides up to three times sharper lateral penumbra.
Mevion claims that the MEVION S250i is "the most technologically advanced and financially viable choice in proton therapy."
"This has been achieved by reducing the footprint to less than half of other single room systems, which directly reduces construction costs and installation timelines," said Tajima.
Zuid-Oost Nederland Protonen Therapie Centrum at the Maastro Clinic in Maastricht, the Netherlands will be the first in Europe to install the MEVION S250i.
In September, Mevion
submitted its 510(k) Premarket Notification to the FDA for the MEVION S250i with HYPERSCAN technology. Mevion CEO Joe Jachinowski said at the time that it's possible that it may receive clearance in 90 days or less.

