by
John R. Fischer, Senior Reporter | February 06, 2018
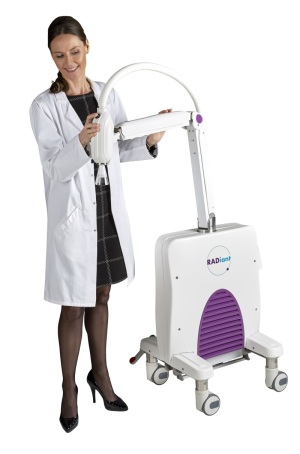
Xstrahl's RADiant therapy system
RADiant therapy system, manufactured by X-ray technology designer Xstrahl, is now available to physicians across the U.S., following its launch on January 31.
The compact radiotherapy system is designed with a dual modality that makes it a pain-free, non-surgical alternative for treating non-melanoma skin cancers, keloids, and superficial lesions, especially ones in hard to reach or sensitive facial regions.
“If a patient is presented with a non-melanoma skin cancer, their options are usually going to be surgery or no surgery. Depending on depth of cancer, you can be left with quite a scar, which might need some attention from a plastic surgeon,” Adrian Treverton, Xstrahl Group CEO, told HCB News. “What our system will do is [deliver] radiation that knocks out the cancer cells, which are less radioresistant than normal tissue. It actually means that the patient will heal up without any scarring at all. Physicians can remove keloids and deliver radiation that stops the keloids from growing back.”



Ad Statistics
Times Displayed: 68410
Times Visited: 2225 Ampronix, a Top Master Distributor for Sony Medical, provides Sales, Service & Exchanges for Sony Surgical Displays, Printers, & More. Rely on Us for Expert Support Tailored to Your Needs. Email info@ampronix.com or Call 949-273-8000 for Premier Pricing.
Surgery often leaves scarring, while no surgery still requires small incisions, making the procedure minimally invasive with risk of scarring. Keloids can often only be treated with painful injections.
RADiant treatments feature efficient cosmesis and low recurrence, enabling patients to undergo procedures while still living active lives.
The low energy of the system and high dose rates of its dose profile also keep treatment time to a minimum.
The solution is lightweight, maneuverable in design, and comes with plug-in operation. It is equipped with advanced Concerto control software, which links to EMRs, enhancing accessibility for patients.
“It’s a lot smaller and more compact and maneuverable than any other radiotherapy systems that are out there right now,” said Treverton. “It also has the ability to deliver both superficial radiotherapy and electronic brachytherapy with the different applicators that we can display with the unit.”
The system is FDA-cleared for use, and will be on display on Friday, February 16 at the 2018 Annual Meeting of the American Academy of Dermatology in San Diego.
Xstrahl is currently seeking a CE mark designation for the product.

