by
John R. Fischer, Senior Reporter | May 16, 2018
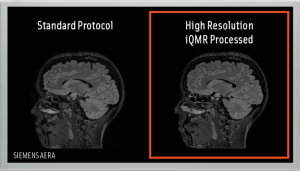
The image acquired and post-processed
by iQMR has 20 percent higher
resolution than the one that was
produced by another solution
Routine brain MR scans may soon take place in less time while still producing top image quality, without the need for repeat scans, following the FDA’s greenlighting Medic Vision Imaging Solution Ltd.’s intelligent Quick MR solution (iQMR).
The iterative image reconstruction technology, along with a clinical study that recorded a 30 percent reduction in exam time for routine brain scans, is set to make its debut next month at the ASNR 56th Annual Meeting in Vancouver, British Columbia, Canada.
“Brain and Spine are the most common MR exams, and the scan time is typically 15 - 25 minutes. Long scan time has several important implications, including patient discomfort and anxiety due to the need to remain motionless inside the small and very loud MR bore,” Eyal Aharon, CEO of Medic Vision Imaging Solutions, told HCB News. “Longer scans result in more motion artifacts due to patient discomfort, which grows with the scan time. This results in the need to repeat certain portions of the scan, making the scan even longer.”



Ad Statistics
Times Displayed: 656
Times Visited: 5 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
A recent research study found that 68 percent of MR patients expressed medium or high levels of anxiety during the time it took to perform an MR exam.
iQMR enhances noisy MR images and increases signal-to-noise ratio, allowing for short MR protocols to take place. This raises productivity, promotes patient satisfaction and experience, and reduces the need for repeat scans.
In reducing repeat scanning, iQMR saves providers on expenses while maintaining existing workflow. A July 2015 report by the ACR found that repeat MR scans due to patient motion cost providers a loss of up to $100,000 in annual revenue.
“The cost of the MR scanner and its limited throughput due to long scans drives MR operators to try and squeeze as many patients in as possible during a workday,” said Aharon. “iQMR can ease the stress on the MR operators – they can keep schedule without compromising scan quality.”
Clearance for iQMR coincides with the two-year mark of clearance for the enterprise’s SafeCT-29, the first third-party XR-29 solution to receive a nod from the FDA. The add-on system enables providers to equip older CT scanners with the ability to meet the requirements that come with XR-29 certification for greater patient safety while saving on the costs associated with newer scanners.
XR-29 compliance includes:
• Automatic exposure control (AEC) to access radiation dose in real time
• Built-in adult and pediatric protocols for the operator to choose from
• Dose Check for automatic notification of when scan settings are likely to exceed dose thresholds
• DICOM SR to incorporate dose history into the patient record
iQMR is supportive of all MR scanner models produced by any vendor and can be used to assess scans of other anatomical parts, including the spine and abdomen.
The solution is now available for sale.

