by
John R. Fischer, Senior Reporter | January 24, 2019
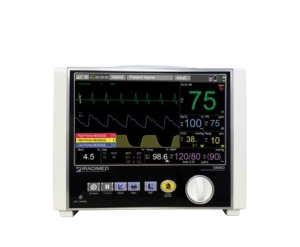
3880 MRI compatible patient vital
signs monitoring system
Shipments of the 3880 MR-compatible patient vital signs monitoring system by IRADIMED have halted following the expiration of its CE Mark this month.
The suspension comes amidst a notification from UL International, which oversees the regulation and CE marking of products in Europe, informing the company that the product does not meet specifications outlined in newly issued guidelines by the European Union.
"We are fully cooperating and in direct discussions with UL to agree upon the necessary and proper application of the new guidance,” said Roger Susi, president and chief executive officer of IRADIMED, in a statement. “We believe that data collection, documentation and UL's review of the required information will take between three to four months, after which time we believe UL will renew the EC Certificate, once again permitting use of the CE Mark on our MR-compatible patient vital signs monitoring system.



Ad Statistics
Times Displayed: 137929
Times Visited: 7957 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
Originally cleared by UL and added to the company’s CE certificate in June 2017, the 3880 MR-compatible patient vital signs monitoring system is designed to accurately track and monitor patient vital signs during MR procedures, and is capable of operating virtually anywhere in an MR room, with magnetic fields of up to 30,000 gauss.
The technical file of the product was subsequently re-reviewed by UL, which notified IRADIMED on January 16 that such a review could not be completed due to the solution not meeting aspects of clinical evaluation reporting, a requirement outlined in newly issued EU guidelines, creating a technical non-conformity.
The company is cooperating with UL to solve the non-conformity, and in full compliance, has suspended shipments of the monitor to all markets that require a CE mark. It maintains that the expiration is not related to any safety, effectiveness or performance issues with the system and will impact neither sales in the U.S. and other non-European Commission markets, nor the delivery of its MR-compatible IV infusion pump and related accessories, disposables or services.
"We expect this action may reduce full-year 2019 revenue by approximately two percent,” said Susi. “After considering this impact, we still expect mid 20 percent revenue growth in 2019. We will provide our full-year 2019 revenue, GAAP, and non-GAAP earnings guidance in our fourth quarter earnings release.”
UL has issued a temporary EC Certificate that excludes the 3880 patient vital signs monitoring system and gives the company six months to solve the non-conformity issue and regain CE marking for the solution.
The matter is expected to be fully resolved before the end of the second quarter.

