by
Joan Trombetti, Writer | February 20, 2008
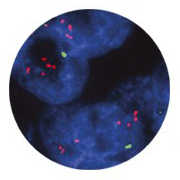
Breast carcinoma
stained with
TOP2A FISH pharmDx
A genetic test used to assess the risk of tumor recurrence and long-term survival for women with aggressive breast cancer has been approved by the FDA. TOP2AFISH pharmDX is the first approved device to test for the TOP2A (topoisomerase-2-alpha) gene in cancer patients.
The test uses fluorescent in situ hybridization (FISH) to detect abnormalities in TOP2A (a gene that plays a role in DNA replication). When used in conjunction with clinical examination and other laboratory tests, the genetic assay can refine prognosis according to Daniel Schultz, MD, director of the FDA's Center for Devices and Radiological Health.
The FDA said the study validated the test for use in estimating time to local or distant recurrence as well as overall survival.



Ad Statistics
Times Displayed: 69864
Times Visited: 2294 Ampronix, a Top Master Distributor for Sony Medical, provides Sales, Service & Exchanges for Sony Surgical Displays, Printers, & More. Rely on Us for Expert Support Tailored to Your Needs. Email info@ampronix.com or Call 949-273-8000 for Premier Pricing.
Read the FDA announcement at:
http://www.fda.gov/cdrh/mda/docs/P050045.html

