bone morphogenetic
protein
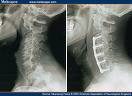
The U.S. Food and Drug Administration warned that bone growth products made by Medtronic, Inc. and Stryker Corp. have been linked to life-threatening complications when used without approval in neck fusion surgeries.
The FDA reported it has received at least 38 complaints in the last four years of neck swelling and breathing problems in patients given the products, which are not FDA-cleared for use in the neck.
Medtronic and Stryker make types of recombinant human bone morphogenetic proteins used to help treat degenerative disc disease, fractures and other bone conditions. InFuse Bone Graft by Medtronic and OP-1 Implant and OP-1 Putty by Stryker both contain such protein.



Ad Statistics
Times Displayed: 109208
Times Visited: 6638 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
Daniel Schultz, head of the FDA's device center, said it was not clear what caused the complications or whether certain patients were at greater risk. He said the neck's close proximity to critical airways was part of the problem.
In some cases, patients had to have a tube inserted to help them breathe or had to have a tracheotomy. Others were given medications or underwent further surgery.
Most of the complications arose between two days and two weeks after surgery, Schultz said in a letter to doctors posted on the agency's website.
In response to the FDA notice, Stryker Corporation responded, "OP-1 is not indicated for use as a bone graft substitute in the cervical spine and healthcare professionals should follow the approved clinical indication for this and all of our products."

