by
John R. Fischer, Senior Reporter | September 18, 2017
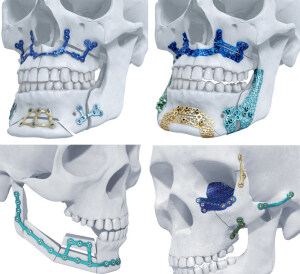
TRUMATCH CMF Titanium 3-D
Printed Implant System
DePuy Synthes will begin rolling out its TRUMATCH CMF Titanium 3-D Printed Implant System on to the U.S. market this month.
The FDA-approved system will be up for sale to consumers starting in mid-September, offering the first patient specific, 3-D printed titanium implants for facial reconstruction using additive manufacturing of titanium as well as the first patient specific implants approved for orthognathic surgery. Distribution will be carried out exclusively by Materialise.
“This is the next step in personalized health care, providing implants that help the surgeon transfer his or her preoperative plan to the operating room, further improving the surgical accuracy and efficiency that they have already experienced for the last five years with our existing TruMatch CMF virtual planning and patient-specific surgical guides,” Michael Barthold, senior group manager of TruMatch Trauma/CMF R&D at DePuy Synthes, told HCB News.




Ad Statistics
Times Displayed: 44472
Times Visited: 1374 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
Current implants that are designed specifically for individual patients are manufactured using subtractive production methods, such as CNC machining or contouring by the device company to create a match with the patient’s anatomy.
The TRUMATCH CMF Titanium 3-D Printed Implants are formed using a CT scan of a patient’s skull as a guide along with the outcome of computer-aided surgical planning. This provides increased accuracy for surgeons and better patient satisfaction.
Its release also allows DePuy Synthes the opportunity to offer TRUMATCH Orthognathics, a personalized solution for corrective jaw surgery.
The U.S. Department of Defense awarded DePuy Synthes in May with a
national contract for its orthopaedic products.
The system is approved for use in Europe, Australia and parts of the Asia-Pacific region.

