by
Thomas Dworetzky, Contributing Reporter | March 20, 2017
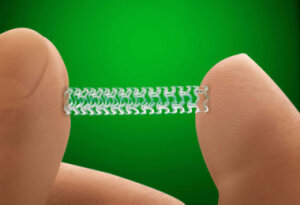
New concerns about the Absorb bioresorbable scaffold stent have come to light in two-year results of the ABSORB III trial, researchers reported at a clinical trials session at the American College of Cardiology’s 66th Annual Scientific Session, March 18.
“Patients receiving Absorb BVS faced an overall elevated risk of adverse outcomes at two years compared with patients receiving metal stents, a difference that appears to be attributable to the stent being placed in vessels that were smaller than recommended,” Absorb-maker Abbott said in a statement.
The FDA has sent a letter about the issue to cardiologists and health care providers. The agency stated to those treating patients with the Absorb GT1 Bioresorbable Vascular Scaffold (BVS) that there is “an increased rate of major adverse cardiac events observed in patients receiving the BVS, when compared to patients treated with the approved, metallic XIENCE drug-eluting stent.”



Ad Statistics
Times Displayed: 2940
Times Visited: 30 Fast-moving cardiac structures have a big impact on imaging. Fujifilm’s SCENARIA View premium performance CT brings solutions to address motion in Coronary CTA while delivering unique dose saving and workflow increasing benefits.
It specified that the findings showed “an 11 percent rate of major adverse cardiac events (e.g., cardiac death, heart attack, or the need for an additional procedure to reopen the treated heart vessel) in patients treated with the BVS at two years, compared with 7.9 percent in patients treated with the already-approved Abbott Vascular's metallic Xience drug-eluting stent,” noting, in addition, that the review also found “a 1.9 percent rate of developing blood clots within the BVS versus 0.8 percent within the Xience stent at 2 years.”
The FDA's guidelines advised “avoiding its use in small vessels.”
Cleveland Clinic cardiologist and the study's lead author Dr. Stephen Ellis called small-vessel avoidance a “key take-home” in the Abbott statement, adding that, "this isn't necessarily surprising, because the Absorb BVS is considerably larger than the Xience stent, but it would have been preferable to have more consistency with regard to the vessel sizing and utilization of the device in the study."
One-year results had shown absorbable stents were “non-inferior” compared with metal ones “as measured by a composite of cardiac death, target lesion heart attack and ischemia-driven target lesion revascularization.”
This changed between year one and two, however, as the latest report showed, according to Abbott. These new results demonstrated “that at the end of year two, patients receiving Absorb BVS showed a significantly higher risk of target lesion failure,” although there were some “inconsistencies with regard to adherence to study protocols and the procedural techniques used to place the device” that may have led to some of the adverse outcomes.