by
John W. Mitchell, Senior Correspondent | March 18, 2016
In the past three years, endoscope cleaning practices have killed 21 patients and sickened hundreds at U.S hospitals. ECRI conducted a webinar attended by more than 300 of its members to learn more about issue number one on its “2016 Top 10 Health Technology Hazards” list.
Three experts - an engineer, a Ph.D. and a nurse - presented the latest in findings and recommendations Wednesday in a webinar titled, “The Reprocessing of Flexible Endoscopes – Are We Doing Enough to Protect Patients?” According to stats shared in the session, there are about 20 million gastroenterology endoscopic procedures conducted annually in the U.S.
“It came as a surprise to those investigating contamination that (hospital) staffs doing the reprocessing were often following the manufacturers process to the letter,” Chris Lavanchy, Engineering Director at ECRI and one of the presenters told HCB News. “The problem has not gone away.”



Ad Statistics
Times Displayed: 123124
Times Visited: 7135 MIT labs, experts in Multi-Vendor component level repair of: MRI Coils, RF amplifiers, Gradient Amplifiers Contrast Media Injectors. System repairs, sub-assembly repairs, component level repairs, refurbish/calibrate. info@mitlabsusa.com/+1 (305) 470-8013
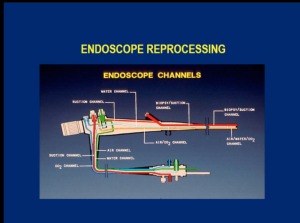
Dr. William Rutala, Ph.D. and Director of Hospital Epidemiology and Occupational Health and Safety at the University of North Carolina School of Medicine noted that by their nature, scopes - especially duodenoscopes - are problematic, compared to other surgical instruments. He explained that scopes have channels, pitted surfaces, bends, springs and levers that can harbor up to 10 billion different gastroenterological microbial organisms, compared to about 100 different organisms found on used general-use surgical instruments.
Contamination risk, which can remain when a scope is properly cleaned according to manufacture's specification, is compounded when recommended procedures are not followed. Rutala said this could happen when sterilization staffs are pushed to get the scopes back into service, often on the same day.
Michelle Day, MSN and a Certified Gastroenterology Nurse, who is the Team Leader at the Comprehensive Liver Center at Hartford Hospital, Connecticut, told the audience that hospitals have to be ready to spend money for increased staffing, education and scope inventory to address the contamination threat.
“You need more staff beyond the technicians, who are already pressured,” Day stressed. She said in addition to reorganizing (and adding a tech) into a “buddy system” they also funded a full-time employee in the laboratory for scope cleanliness testing.
Rutala, who along with a physician colleague published a paper on the problem in JAMA in 2014, reviewed “enhanced methods” for reprocessing duodenoscopes. These include using ethylene oxide sterilization (ETO), deploying double disinfections, quarantining scopes for follow-up culturing and other measures.