by
Gail Kalinoski, Contributing Reporter | March 16, 2016
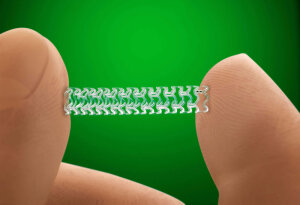
Abbott’s dissolving heart stent – Absorb – received a positive review this week from the FDA advisory panel that ruled the device’s benefits outweigh the risks.
The panel of independent experts reviewed clinical data about the safety and efficacy of Absorb, the first bioresorbable device for treatment of coronary artery disease, compared to the leading metallic drug-eluting stent that is a permanent implant. Absorb, made of a naturally dissolvable material, dissolves completely within two to three years after it has kept a clogged artery open long enough to heal.
“Fully dissolvable devices represent a transformative advance in the treatment of coronary artery blockages,” Dr. Charles Simonton, chief medical officer and divisional vice president of medical affairs for Abbott’s vascular business, said in a statement. “The unique benefit of Absorb is that it opens the blockage like a metal stent, but then goes away over time, allowing the artery to return to a more natural state. That makes the Absorb stent a very attractive option for many patients who don’t want permanent implants inside their arteries for the rest of their lives.”




Ad Statistics
Times Displayed: 27892
Times Visited: 627 Stay up to date with the latest training to fix, troubleshoot, and maintain your critical care devices. GE HealthCare offers multiple training formats to empower teams and expand knowledge, saving you time and money
Simonton thanked the panel, which met Tuesday outside Washington, D.C., for its thorough review of the data presented. On the question of the device’s safety as a treatment for coronary artery disease, the panel voted 9 to 1 in favor, with one abstention. On a separate question of the device’s efficacy, the panel unanimously said yes.
“We look forward to continuing discussions with the FDA on our submission for approval of this device in the U.S.,” he said.
Approximately 850,000 patients in the United States receive heart stents every year,
according to the Press Enterprise newspaper in Temecula, Calif., where Abbott manufactures Absorb.
Abbott submitted its pre-market approval (PMA) application to the FDA in mid-2015. The FDA’s decision on the company’s PMA is expected later this year. It routinely gets input from advisory committees, especially for first-in-kind medical devices, before giving its ruling.
The advisory committee reviewed data from multiple studies, including one U.S. clinical trial with 2,000 people, and compared it to Abbott’s market-leading metallic drug-eluting stent, Xience.
“In multiple randomized clinical trials, the Absorb bioresorbable vascular scaffold has demonstrated comparable outcomes to the leading permanent metallic stent. As a first-in-kind device with novel properties, including complete dissolution and natural restoration of vessel function, this is a remarkable achievement,” said Dr. Gregg Stone, director, cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia Medical Center, New York Presbyterian Hospital. Stone is also the chairman of the Absorb clinical trial program.