by
John R. Fischer, Senior Reporter | May 15, 2020
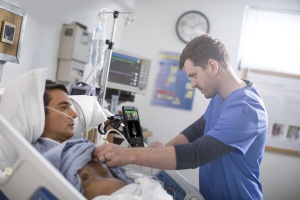
The Lumify with Reacts handheld tele-ultrasound solution is one of a number of ultrasound products by Philips that the FDA has approved for providers to use in the fight against the COVID-19 pandemic.
The FDA has given the green light for providers to manage COVID-19-related lung and cardiac complications with a number of Philips’ ultrasound solutions.
The regulatory clearance is an industry first according to the Dutch healthcare tech giant, which is providing detailed, practice guidance to instruct clinicians on the use of its approved systems and software.
"Philips has a comprehensive portfolio of products, services and solutions to help healthcare providers deliver high-quality care to COVID-19 patients ranging from diagnosis, treatment and monitoring in the hospital, to screening and monitoring at home," Bich Le, senior vice president and general manager of ultrasound at Philips, told HCB News. "Our Lumify with Reacts handheld tele-ultrasound solution is being used as a valuable 'over the shoulder' education platform for new users in critical care and lower resource settings, who can get help and guidance from more experienced users."



Ad Statistics
Times Displayed: 616
Times Visited: 3 Keep biomedical devices ready to go, so care teams can be ready to care for patients. GE HealthCare’s ReadySee™ helps overcome frustrations due to lack of network and device visibility, manual troubleshooting, and downtime.
Among the products approved for use are the EPIQ series, Affiniti series, Lumify, CX50 and Sparq diagnostic ultrasound systems, as well as off-cart solutions such as QLAB Advanced Quantification Software.
The clearances are tied to the fact that ultrasound is valuable in imaging peripheral lung tissue affected by pneumonia, which is closely related to COVID-19 lung complications. Respiratory strain can also lead to cardiac dysfunction, putting patients at risk for cardiac complications and in need of cardiac ultrasound exams to monitor the impact of the disease on heart function, says Philips.
The solutions will enable clinicians to image COVID-19 patients at the point of care, and help them diagnose and monitor patients without having to move them around the hospital. This reduces the risk of virus transmission to other patients and to healthcare practitioners.
Philips’ guidelines highlight specific presets, transducers, quantification tools and other capabilities available on its systems for assessing COVID-19-related lung and cardiac complications. For instance, the EPIQ CVx premium cardiology ultrasound system offers automated applications for 2D assessment of the heart, as well as robust 3D right ventricle volume and ejection fraction measurements.
Philips, like many other healthcare companies, has focused its efforts on providing necessary technologies and supplies to providers since the beginning of the pandemic. The company recently
unveiled its Philips Respironics E30 ventilator, a versatile solution designed for noninvasive and invasive ventilation, and intends to produce its whole stock of ventilators at a rate of 4,000 units per week by the third quarter of 2020. It plans to increase its supply of ultrasound solutions as well.
"We're also helping to connect physicians around the world through best practice webinars," said Le. "For example, we recently hosted an online event that included physicians from Wuhan in China, Italy, and several other countries."

